Label: MESALAMINE capsule, delayed release
- NDC Code(s): 0093-5907-86
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MESALAMINE DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for MESALAMINE DELAYED-RELEASE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Mildly to Moderately Active Ulcerative Colitis - Mesalamine delayed-release capsules are indicated for the treatment of mildly to moderately active ulcerative colitis in patients ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Do not substitute two mesalamine delayed-release 400 mg capsules with one mesalamine delayed-release 800 mg tablet. Evaluate renal function prior to ...
-
3 DOSAGE FORMS AND STRENGTHSMesalamine delayed-release capsules are clear capsules imprinted with “TEVA” and “5907” on both the cap and the body in black ink. Each capsule contains four reddish-brown, film-coated round 100 ...
-
4 CONTRAINDICATIONSMesalamine delayed-release capsules are contraindicated in patients with known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of mesalamine ...
-
5 WARNINGS AND PRECAUTIONS5.1 Renal Impairment - Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure, has been reported in patients taking products such as ...
-
6 ADVERSE REACTIONSThe most serious adverse reactions seen in mesalamine clinical trials or with other products that contain or are metabolized to mesalamine are: Renal Impairment [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs - The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well controlled studies of mesalamine use in pregnant women. Limited published human data on mesalamine show no increase in the overall ...
-
10 OVERDOSAGEMesalamine delayed-release capsules is an aminosalicylate, and symptoms of salicylate toxicity include nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms ...
-
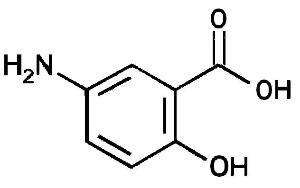
11 DESCRIPTIONEach Mesalamine delayed-release capsule for oral administration contains four 100 mg tablets of mesalamine, an aminosalicylate. Mesalamine delayed-release capsules contain acrylic based resin ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of mesalamine is not fully understood, but appears to be a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Mesalamine was not carcinogenic at dietary doses of up to 480 mg/kg/day in rats and 2000 mg/kg/day in mice, which are ...
-
14 CLINICAL STUDIESThe safety and efficacy of mesalamine has been established based on adequate and well-controlled studies of mesalamine delayed-release tablets. Below is a description of the results of the ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMesalamine delayed-release capsules are available as clear capsules imprinted with “TEVA” and “5907” on both the cap and the body in black ink. Each capsule contains four reddish-brown ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Inform patients that if they are switching from a previous oral mesalamine therapy to mesalamine delayed-release capsules to discontinue their previous oral mesalamine therapy ...
-
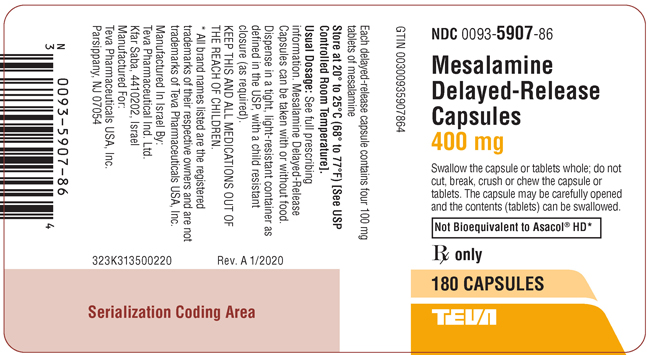
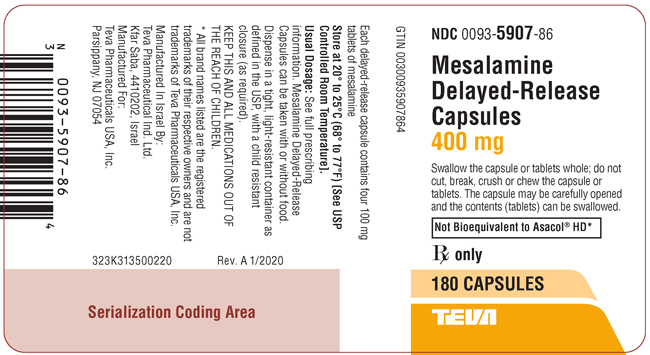
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 0093-5907-86 - Mesalamine Delayed-Release Capsules - 400 mg - Swallow the capsule or tablets whole; do not cut, break, crush or chew the capsule or tablets. The capsule may be carefully opened and ...
-
INGREDIENTS AND APPEARANCEProduct Information