Label: PENICILLIN V POTASSIUM tablet
PENICILLIN V POTASSIUM powder, for solution
- NDC Code(s): 0093-1172-01, 0093-1172-10, 0093-1174-01, 0093-1174-10, view more
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of penicillin V potassium tablets, penicillin V potassium for oral solution, and other antibacterial drugs ...
-
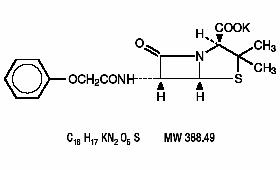
DESCRIPTIONPenicillin V is the phenoxymethyl analog of penicillin G. Penicillin V potassium is the potassium salt of penicillin V. Each Penicillin V Potassium Tablet, USP contains penicillin V potassium ...
-
CLINICAL PHARMACOLOGYPenicillin V exerts a bactericidal action against penicillin-sensitive microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall ...
-
MICROBIOLOGYSusceptibility Testing - For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug ...
-
INDICATIONS AND USAGEPenicillin V potassium tablets and penicillin V potassium for oral solution are indicated in the treatment of mild to moderately severe infections due to penicillin G-sensitive microorganisms ...
-
CONTRAINDICATIONSA previous hypersensitivity reaction to any penicillin is a contraindication.
-
WARNINGSSERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (anaphylactic) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A ...
-
PRECAUTIONSGeneral - Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma. Prescribing penicillin V potassium tablets or penicillin V potassium for ...
-
ADVERSE REACTIONSAlthough the incidence of reactions to oral penicillins has been reported with much less frequency than following parenteral therapy, it should be remembered that all degrees of hypersensitivity ...
-
DOSAGE AND ADMINISTRATIONThe dosage of penicillin V potassium tablets and penicillin V potassium for oral solution should be determined according to the sensitivity of the causative microorganisms and the severity of ...
-
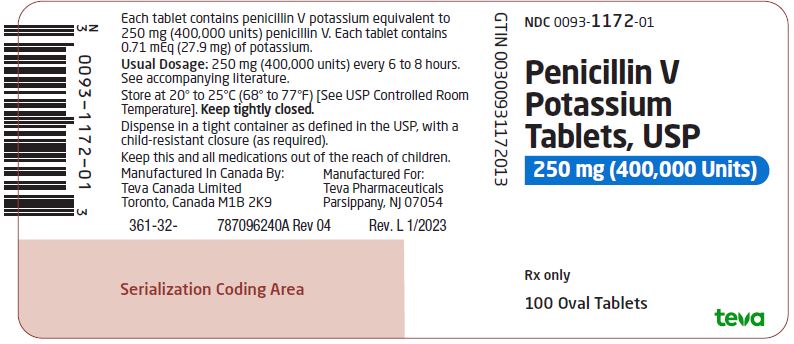
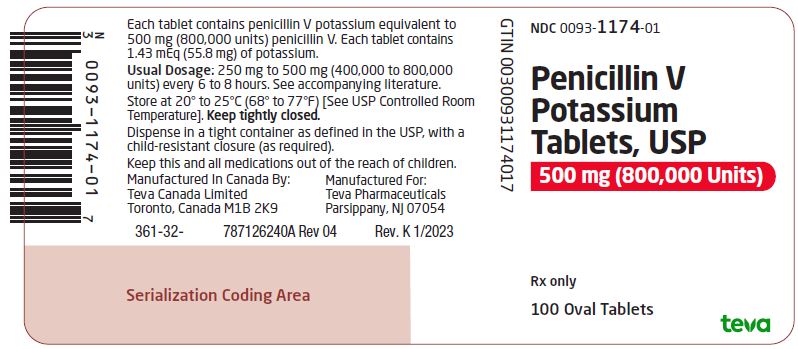
HOW SUPPLIEDPenicillin V Potassium Tablets, USP are available as follows: 250 mg (400,000 units): biconvex, oval, mottled, white to off-white, uncoated tablets, debossed “93” on one side and “1172” on the ...
-
REFERENCESAmerican Heart Association. 1984. Prevention of bacterial endocarditis. Circulation 70 (6): 1123A-1127A. Manufactured In Canada By: Teva Canada Limited - Toronto, Canada M1B 2K9 - Manufactured ...
-
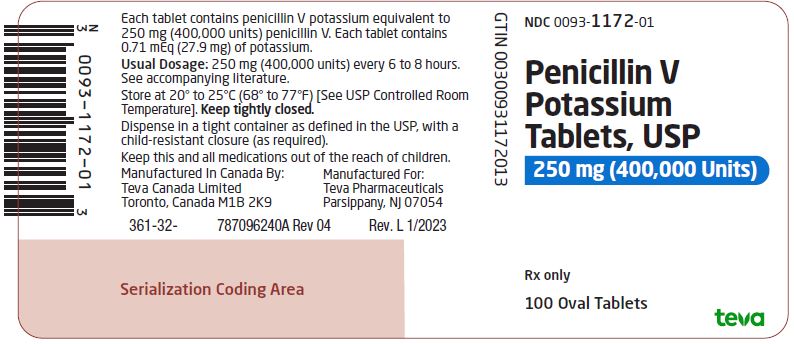
Package/Label Display PanelNDC 0093-1172-01 - Penicillin V - Potassium - Tablets, USP - 250 mg (400,000 Units) Rx only - 100 Oval Tablets
-
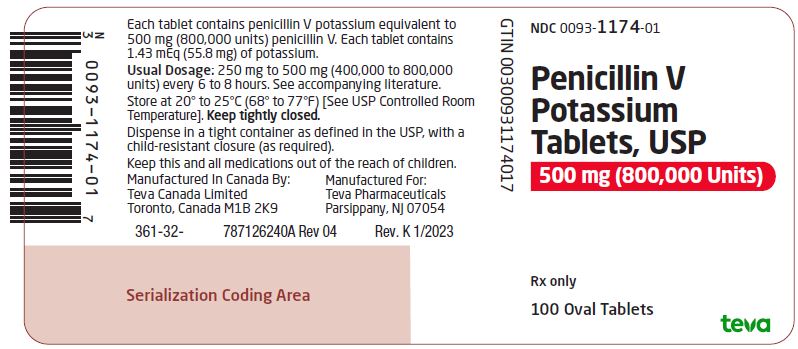
Package/Label Display PanelNDC 0093-1174-01 - Penicillin V - Potassium - Tablets, USP - 500 mg (800,000 Units) Rx only - 100 Oval Tablets
-
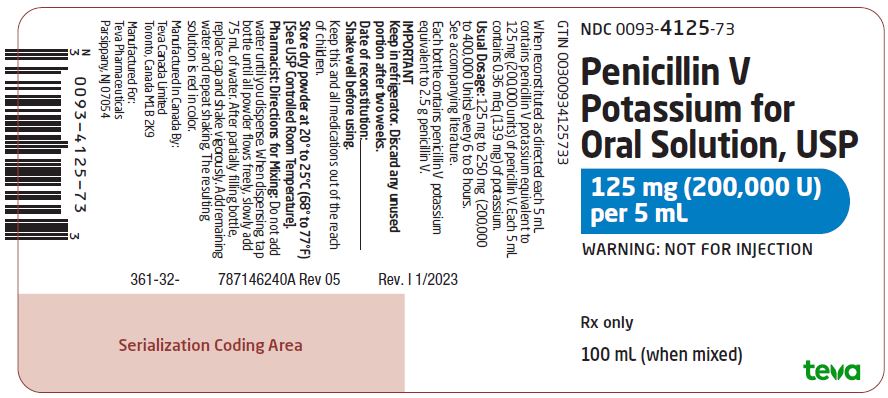
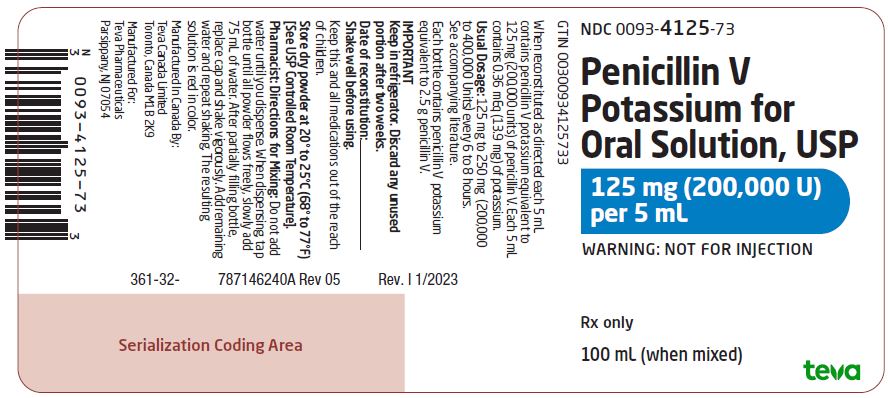
Package/Label Display PanelNDC 0093-4125-73 - Penicillin V - Potassium for - Oral Solution, USP - 125 mg (200,000 U) per 5 mL - WARNING: NOT FOR INJECTION - Rx only - 100 mL (when mixed)
-
Package/Label Display PanelNDC 0093-4127-73 - Penicillin V - Potassium for - Oral Solution, USP - 250 mg (400,000 U) per 5 mL - WARNING: NOT FOR INJECTION - Rx only - 100 mL (when mixed)
-
INGREDIENTS AND APPEARANCEProduct Information