Label: PRAZOSIN HYDROCHLORIDE capsule
- NDC Code(s): 0093-4067-01, 0093-4067-10, 0093-4068-01, 0093-4068-10, view more
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
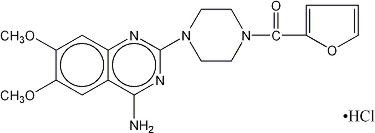
DESCRIPTIONPrazosin hydrochloride, USP a quinazoline derivative, is the first of a new chemical class of antihypertensives. It is the hydrochloride salt of ...
-
CLINICAL PHARMACOLOGYThe exact mechanism of the hypotensive action of prazosin is unknown. Prazosin causes a decrease in total peripheral resistance and was originally thought to have a direct relaxant action on ...
-
INDICATIONS AND USAGEPrazosin hydrochloride capsules USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events ...
-
CONTRAINDICATIONSPrazosin hydrochloride capsules are contraindicated in patients with known sensitivity to quinazolines, prazosin, or any of the inert ingredients.
-
WARNINGSAs with all alpha-blockers, prazosin hydrochloride may cause syncope with sudden loss of consciousness. In most cases, this is believed to be due to an excessive postural hypotensive effect ...
-
PRECAUTIONSGeneral - Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha-1 blockers. This variant of small pupil syndrome is ...
-
ADVERSE REACTIONSClinical trials were conducted on more than 900 patients. During these trials and subsequent marketing experience, the most frequent reactions associated with prazosin hydrochloride therapy are ...
-
OVERDOSAGEAccidental ingestion of at least 50 mg of prazosin in a two-year-old child resulted in profound drowsiness and depressed reflexes. No decrease in blood pressure was noted. Recovery was ...
-
DOSAGE AND ADMINISTRATIONThe dose of prazosin hydrochloride capsules USP should be adjusted according to the patient’s individual blood pressure response. The following is a guide to its administration: Initial Dose - 1 ...
-
HOW SUPPLIEDPrazosin hydrochloride capsules USP are available as follows: 1 mg: An ivory opaque capsule, filled with white powder, imprinted with "TEVA" on the cap and "4067" on the body, containing prazosin ...
-
REFERENCES1. Lubbe, WF, and Hodge, JV: New Zealand Med J, 94 (691) 169-172, 1981. 2. Davey, DA, and Dommisse, J: S.A. Med J, Oct. 4, 1980 (551-556). Distributed By: TEVA PHARMACEUTICALS USA, INC. North ...
-
Package/Label Display PanelPrazosin Hydrochloride Capsules USP 1 mg 100s Label Text - NDC 0093-4067-01 - Prazosin - Hydrochloride - Capsules USP - 1 mg* Rx only - 100 CAPSULES - TEVA
-
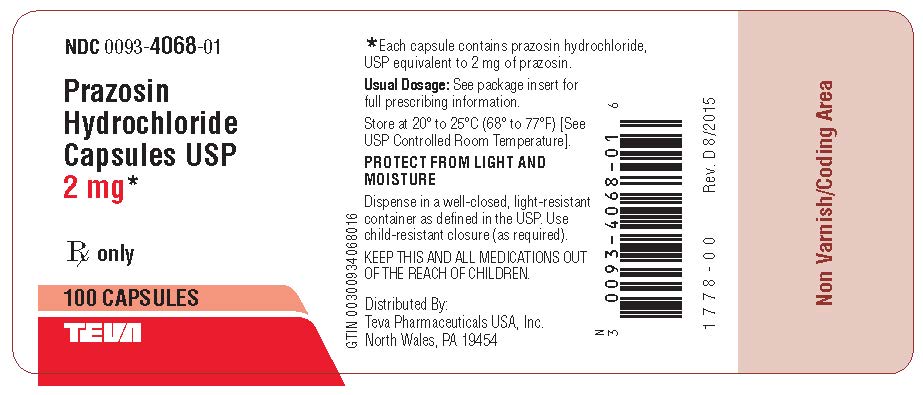
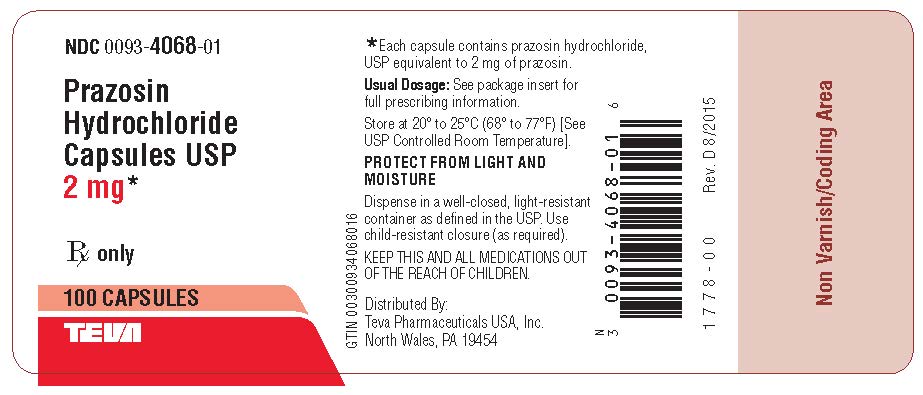
Package/Label Display PanelPrazosin Hydrochloride Capsules USP 2 mg 100s Label Text - NDC 0093-4068-01 - Prazosin - Hydrochloride - Capsules USP - 2 mg* Rx only - 100 CAPSULES - TEVA
-
Package/Label Display PanelPrazosin Hydrochloride Capsules USP 5 mg 100s Label Text - NDC 0093-4069-01 - Prazosin - Hydrochloride - Capsules USP - 5 mg* Rx only - 100 CAPSULES - TEVA
-
INGREDIENTS AND APPEARANCEProduct Information