Label: CEPHALEXIN tablet
- NDC Code(s): 0093-2238-01, 0093-2240-01
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of cephalexin tablets and other antibacterial drugs, cephalexin tablets should be used only to treat or prevent ...
-
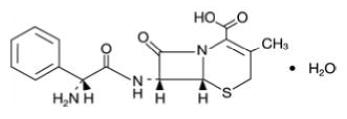
DESCRIPTIONCephalexin tablets, USP contain the active ingredient cephalexin, which is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D-α ...
-
CLINICAL PHARMACOLOGYHuman Pharmacology - Cephalexin tablets are acid stable and may be given without regard to meals. It is rapidly absorbed after oral administration. Following doses of 250 mg, 500 mg, and 1 g ...
-
INDICATIONS AND USAGECephalexin tablets are indicated for the treatment of the following infections when caused by susceptible strains of the designated microorganisms: Respiratory tract infections caused by ...
-
CONTRAINDICATIONSCephalexin tablets are contraindicated in patients with known allergy to cephalexin or other members of the cephalosporin class of antibacterial drugs.
-
WARNINGSHypersensitivity Reactions - Allergic reactions in the form of rash, urticaria, angioedema, anaphylaxis, erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis have been ...
-
PRECAUTIONSGeneral - Prescribing cephalexin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
OVERDOSAGESymptoms of oral overdose may include nausea, vomiting, epigastric distress, diarrhea, and hematuria. In the event of an overdose, institute general supportive measures. Forced diuresis ...
-
DOSAGE AND ADMINISTRATIONCephalexin tablets are administered orally. Adults and Pediatric Patients at Least 15 Years of Age - The usual dose is 250 mg every 6 hours but a dose of 500 mg every 12 hours may be ...
-
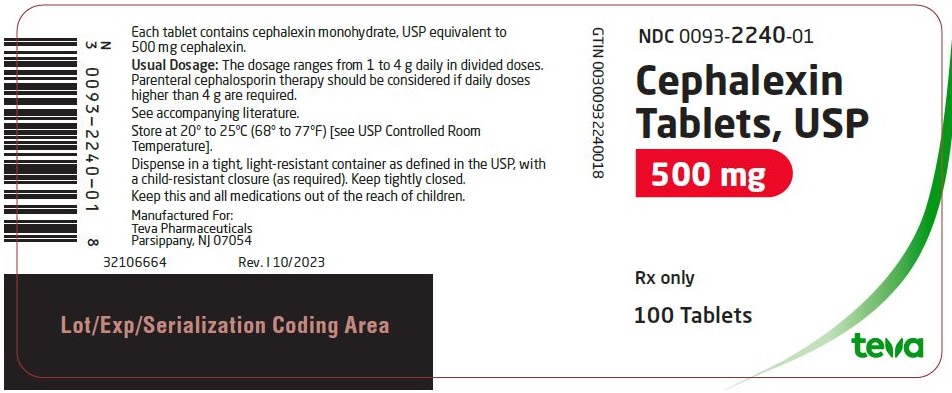
HOW SUPPLIEDCephalexin tablets, USP are supplied as follows: 250 mg: bottles of 100 (NDC 0093-2238-01). Tablet identification number and color: white, capsule-shaped tablet, debossed “2238” on one side with a ...
-
Package/Label Display PanelNDC 0093-2238-01 - Cephalexin Tablets, USP - 250 mg - Rx only - 100 Tablets
-
Package/Label Display PanelNDC 0093-2240-01 - Cephalexin Tablets, USP - 500 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information