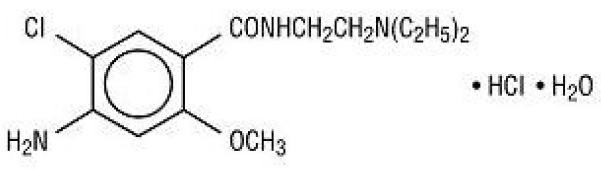Label: METOCLOPRAMIDE tablet
- NDC Code(s): 0093-2203-01, 0093-2203-05, 0093-2203-10, 0093-2204-01, view more
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOCLOPRAMIDE TABLETS safely and effectively. See full prescribing information for METOCLOPRAMIDE TABLETS. METOCLOPRAMIDE tablets ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: TARDIVE DYSKINESIA
- Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage [see Warnings and Precautions (5.1)].
- Discontinue metoclopramide in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after metoclopramide is stopped [see Warnings and Precautions (5.1)].
- Avoid treatment with metoclopramide for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Warnings and Precautions (5.1) and Dosage and Administration (2.2, 2.3)].
-
1 INDICATIONS AND USAGEMetoclopramide tablets are indicated for the: Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy. Relief of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Avoid treatment with metoclopramide for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Dosage and ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 5 mg metoclopramide, USP: white, round, unscored, debossed “TV” on one side and “2204” on the other side. 10 mg metoclopramide, USP: white, round, scored, debossed “TEVA” on one side and ...
-
4 CONTRAINDICATIONSMetoclopramide is contraindicated: In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide [see Warnings and Precautions (5.1, 5.2)]. When stimulation of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Tardive Dyskinesia - Metoclopramide can cause tardive dyskinesia (TD), a syndrome of potentially irreversible and disfiguring involuntary movements of the face or tongue, and sometimes of the ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described, or described in greater detail, in other sections of the labeling: Tardive dyskinesia [see Boxed Warning and Warnings and Precautions (5.1)] Other ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on Metoclopramide - Table 3 displays the effects of other drugs on metoclopramide. Table 3. Effects of Other Drugs on ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published studies, including retrospective cohort studies, national registry studies, and meta-analyses, do not report an increased risk of adverse ...
-
10 OVERDOSAGEManifestations of metoclopramide overdosage included drowsiness, disorientation, extrapyramidal reactions, other adverse reactions associated with metoclopramide use (including, e.g. ...
-
11 DESCRIPTIONMetoclopramide hydrochloride, USP, the active ingredient of metoclopramide tablets, USP is a dopamine-2 receptor antagonist. Metoclopramide hydrochloride (metoclopramide monohydrochloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. The exact mechanism of action of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A 77-week study was conducted in rats with oral metoclopramide doses up to 40 mg/kg/day (about six times the maximum ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach white, round, unscored, debossed “TV” on one side and “2204” on the other side, compressed metoclopramide tablet, USP contains metoclopramide hydrochloride, USP equivalent to 5 mg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Inform patients or their caregivers that metoclopramide tablets can cause serious adverse reactions. Instruct ...
-
MEDICATION GUIDEDispense with Medication Guide available at: www.tevausa.com/medguides - Metoclopramide (met'' oh kloe' pra mide) Tablets - Read this Medication Guide before you start taking ...
-
Package/Label Display PanelNDC 0093-2204-01 - Metoclopramide Tablets, USP - 5 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
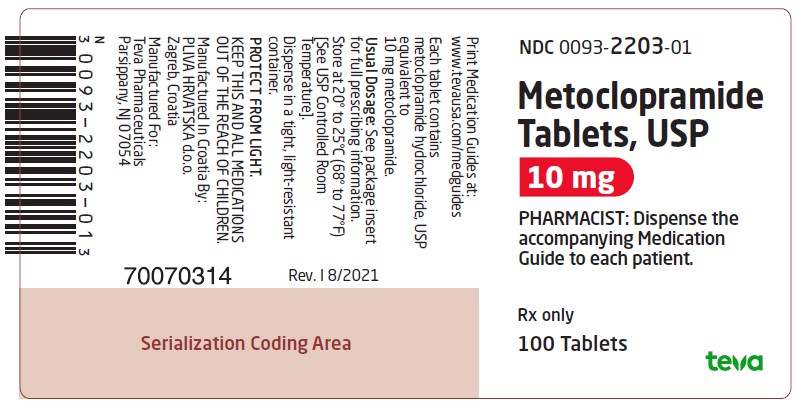
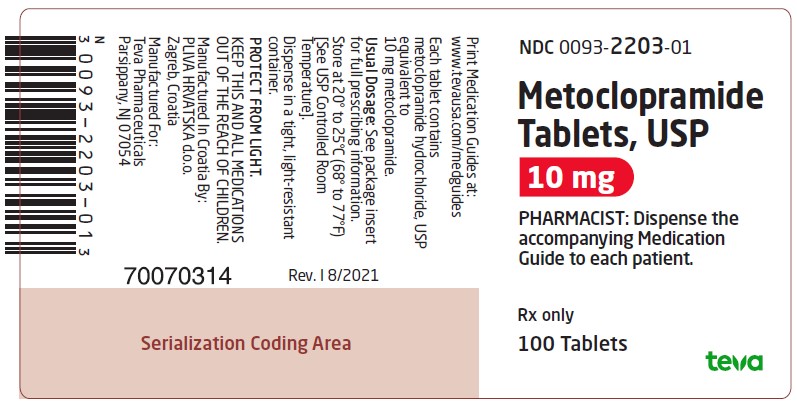
Package/Label Display PanelNDC 0093-2203-01 - Metoclopramide Tablets, USP - 10 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information