Label: MUPIROCIN ointment
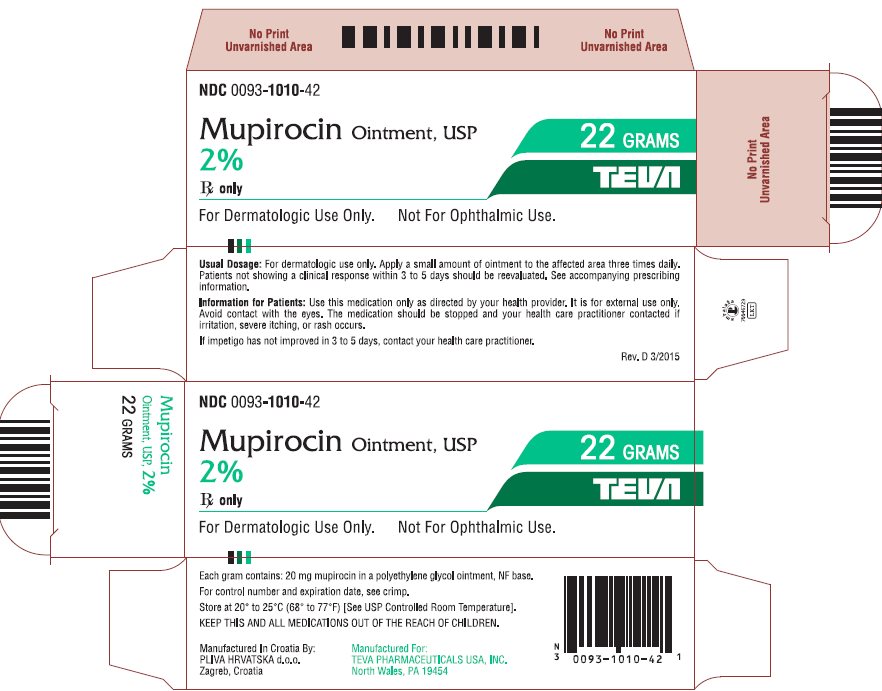
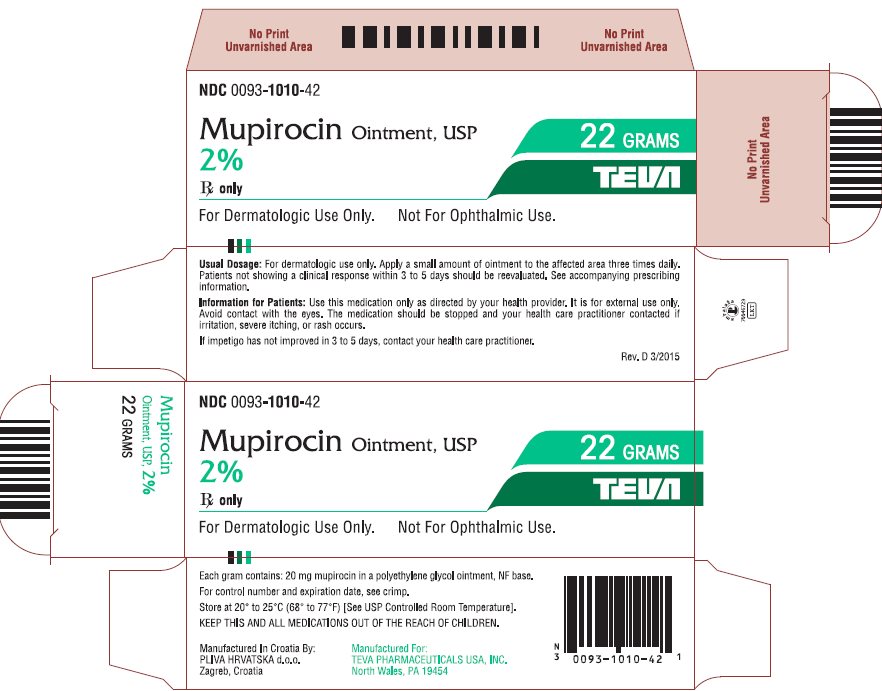
- NDC Code(s): 0093-1010-42
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MUPIROCIN OINTMENT safely and effectively. See full prescribing information for MUPIROCIN OINTMENT. MUPIROCIN ointment, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMupirocin ointment is indicated for the topical treatment of impetigo due to susceptible isolates of Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes).
-
2 DOSAGE AND ADMINISTRATIONFor Topical Use Only. Apply a small amount of mupirocin ointment, with a cotton swab or gauze pad, to the affected area 3 times daily for up to 10 days. Cover the treated area with gauze ...
-
3 DOSAGE FORMS AND STRENGTHSEach gram of mupirocin ointment USP contains 20 mg mupirocin in a water-miscible ointment base supplied in 22-gram tubes.
-
4 CONTRAINDICATIONSMupirocin ointment is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of mupirocin ointment.
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Allergic Reactions - Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Severe Allergic Reactions [see Warnings and Precautions (5.1)] Eye Irritation [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient human data to establish whether there is a drug-associated risk with mupirocin ointment in pregnant women. Systemic absorption of mupirocin ...
-
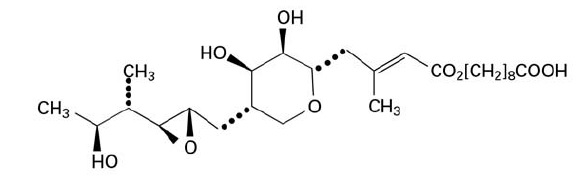
11 DESCRIPTIONMupirocin ointment USP, 2% contains the RNA synthetase inhibitor antibacterial, mupirocin. The chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mupirocin is an RNA synthetase inhibitor antibacterial [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - Application of 14C-labeled mupirocin ointment ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted. Results of the following studies ...
-
14 CLINICAL STUDIESThe efficacy of topical mupirocin ointment in impetigo was tested in 2 trials. In the first, subjects with impetigo were randomized to receive either mupirocin ointment or vehicle placebo 3 times ...
-
15 REFERENCESClinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26. Clinical and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach gram of Mupirocin Ointment USP, 2% contains 20 mg mupirocin in a water-miscible ointment base. Mupirocin Ointment USP, 2% is supplied in 22 gram tubes (NDC 0093-1010-42). Store at 20o to 25oC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Advise the patient to administer mupirocin ointment as follows: Use mupirocin ointment only as directed by ...
-
PATIENT INFORMATIONMupirocin (myu-PIR-ō-sǝn) Ointment USP - What is mupirocin ointment? Mupirocin ointment is a prescription medicine used on the skin (topical use) to treat a skin infection called ...
-
Package/Label Display PanelNDC 0093-1010-42 - Mupirocin Ointment, USP - 2% Rx only - For Dermatologic Use Only. Not For Ophthalmic Use. 22 GRAMS
-
INGREDIENTS AND APPEARANCEProduct Information