Label: PREDNISONE tablet
PREDNISONE solution
PREDNISONE INTENSOL solution, concentrate
- NDC Code(s): 0054-0017-20, 0054-0017-25, 0054-0017-29, 0054-0018-20, view more
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
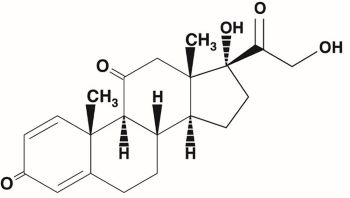
DESCRIPTIONPrednisone is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Prednisone, USP is ...
-
ACTIONS Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic ...
-
INDICATIONSPrednisone tablets and solutions are indicated in the following conditions: 1. Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first ...
-
CONTRAINDICATIONSSystemic fungal infections and known hypersensitivity to components.
-
WARNINGSIn patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is ...
-
PRECAUTIONSGeneral Precautions - Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after ...
-
ADVERSE REACTIONSFluid and Electrolyte Disturbances - Sodium retention - Fluid retention - Congestive heart failure in susceptible patients - Potassium loss - Hypokalemic alkalosis - Hypertension - Musculoskeletal - Muscle ...
-
DOSAGE AND ADMINISTRATION The initial dosage of prednisone may vary from 5 mg to 60 mg of prednisone per day depending on the specific disease entity being treated. In situations of less severity lower doses will generally ...
-
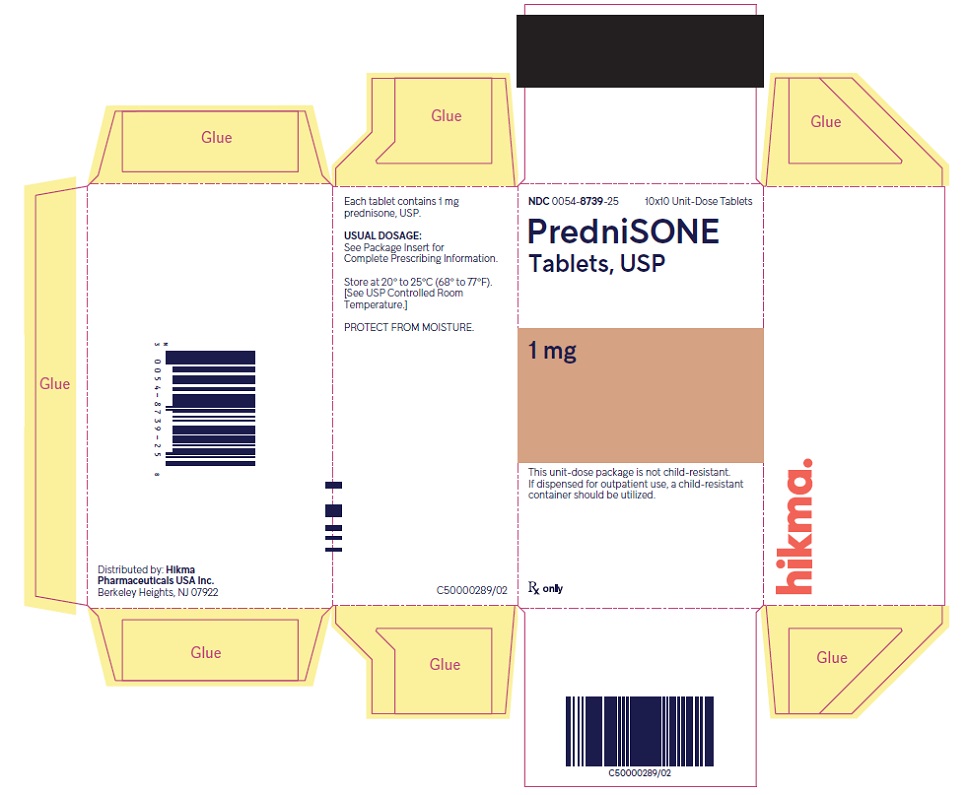
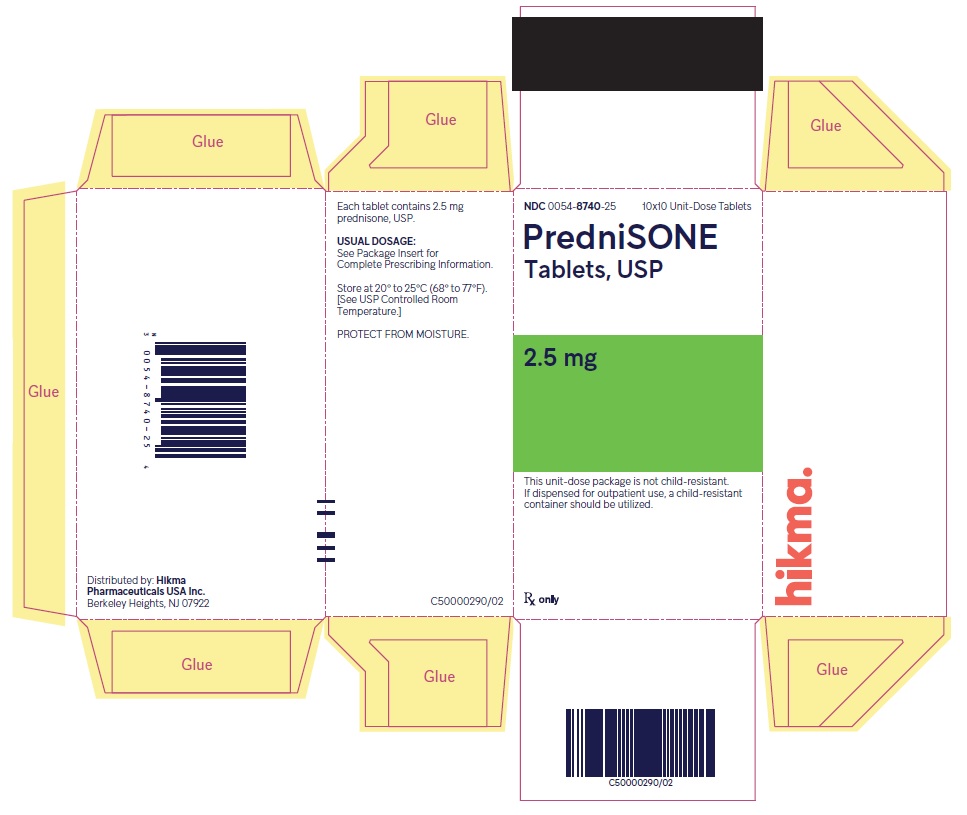
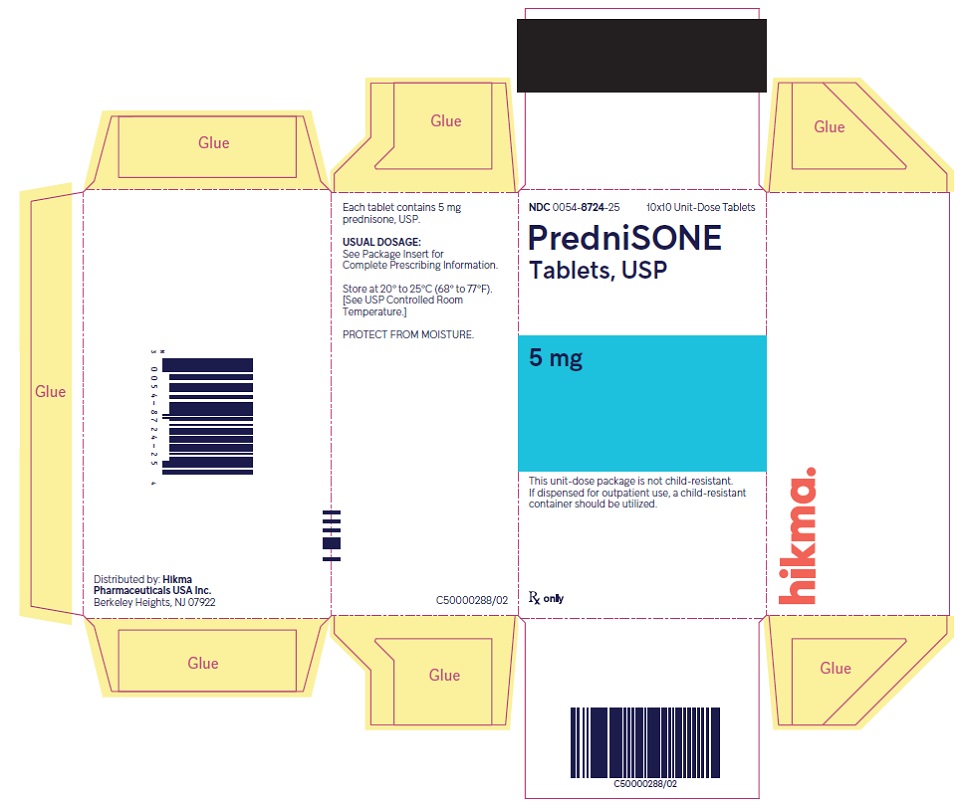
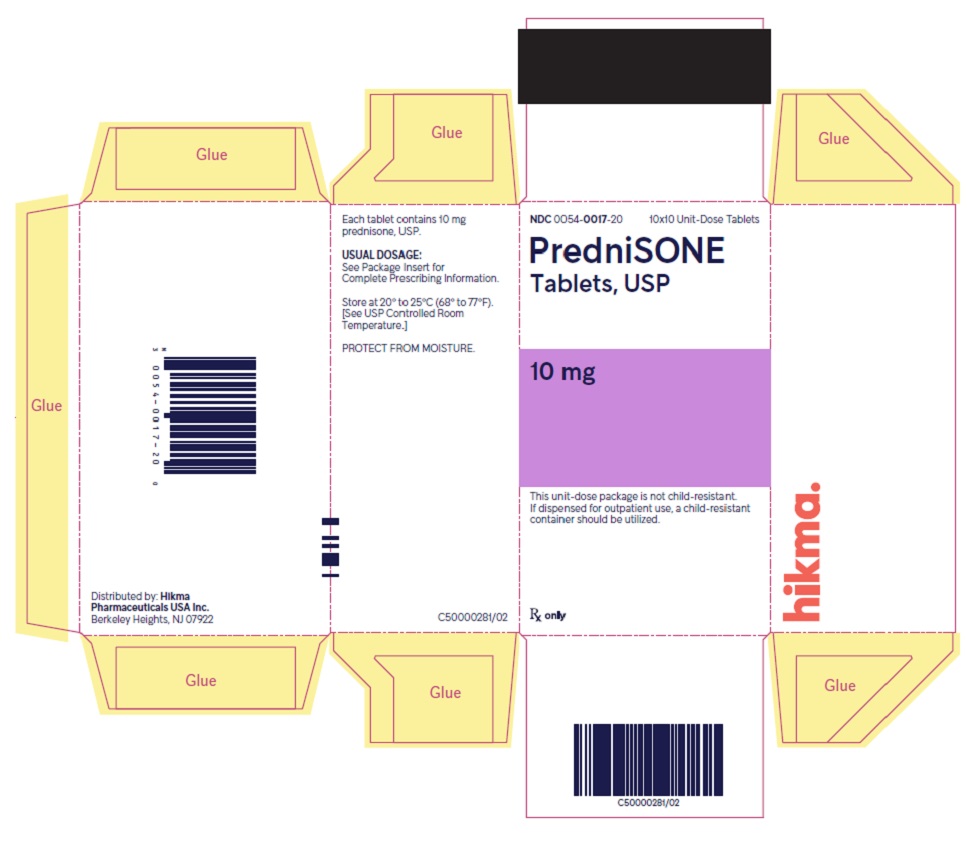
HOW SUPPLIED PredniSONE Tablets, USP - 1 mg – White to off-white, round, biconvex tablet; scored on one side and product identification “54 [above] 092” debossed on the other side. NDC 0054-8739-25: 10x10 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-8739-25 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 1 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-4741-25 100 Tablets - PredniSONE Tablets, USP - 1 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-8740-25 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 2.5 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-4742-25 100 Tablets - PredniSONE Tablets, USP - 2.5 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-8724-25 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 5 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-4728-25 100 Tablets - PredniSONE Tablets, USP - 5 mg
-
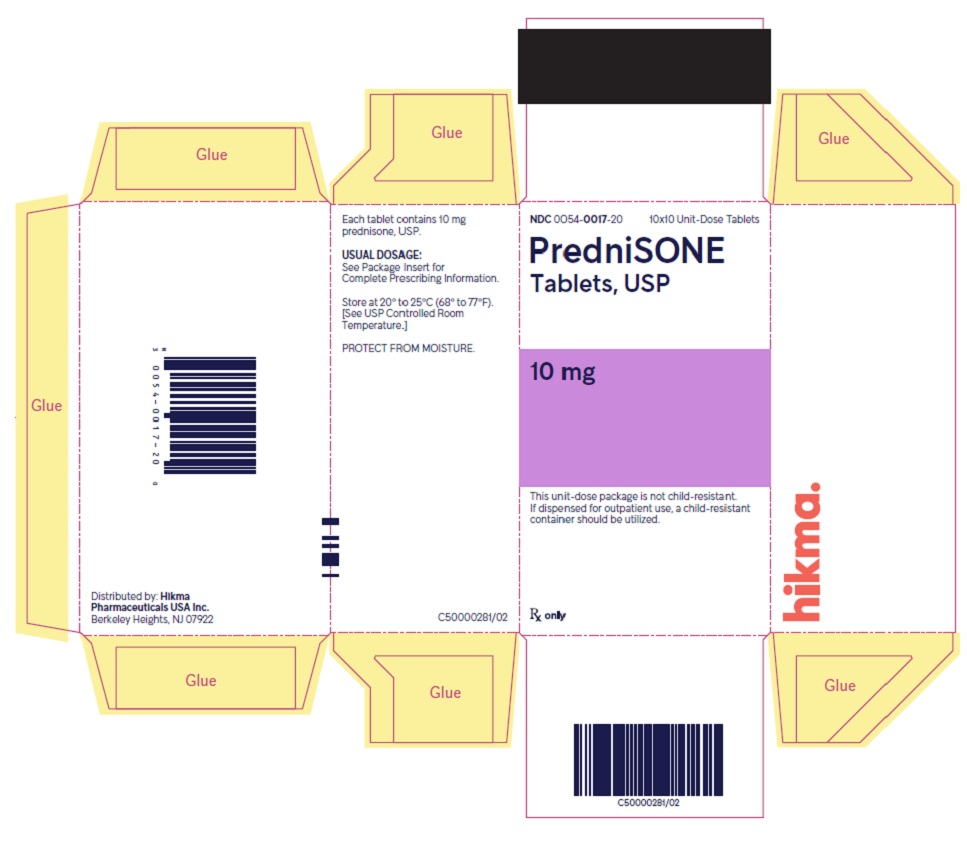
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0017-20 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 10 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0017-25 100 Tablets - PredniSONE Tablets, USP - 10 mg
-
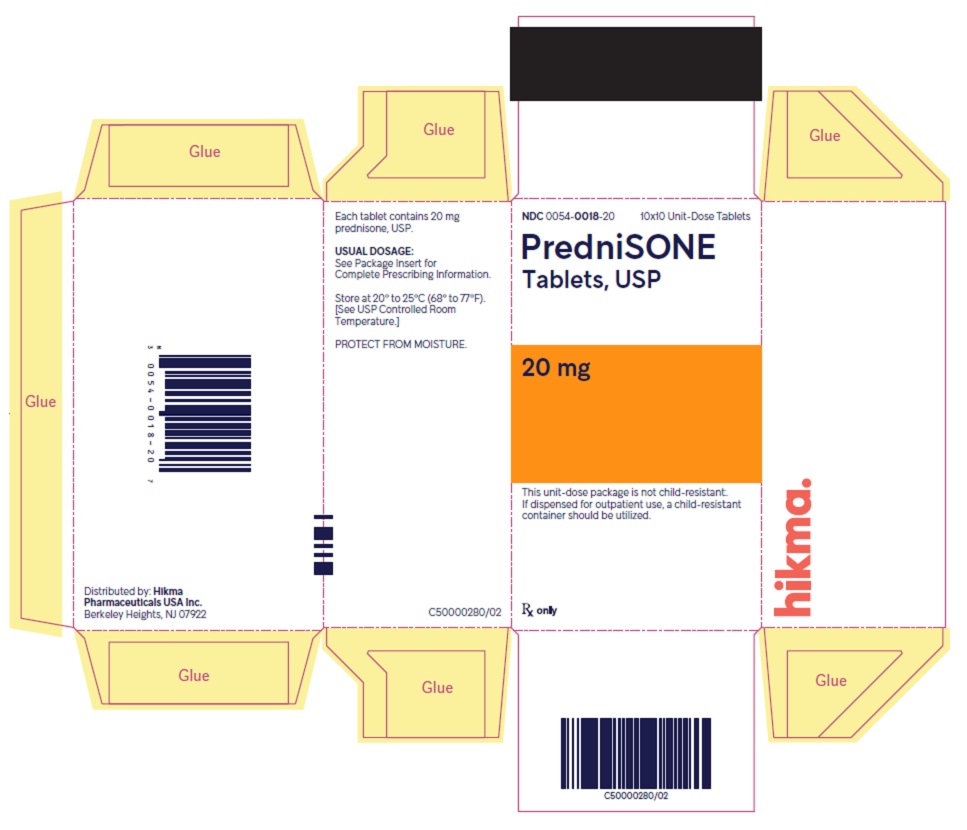
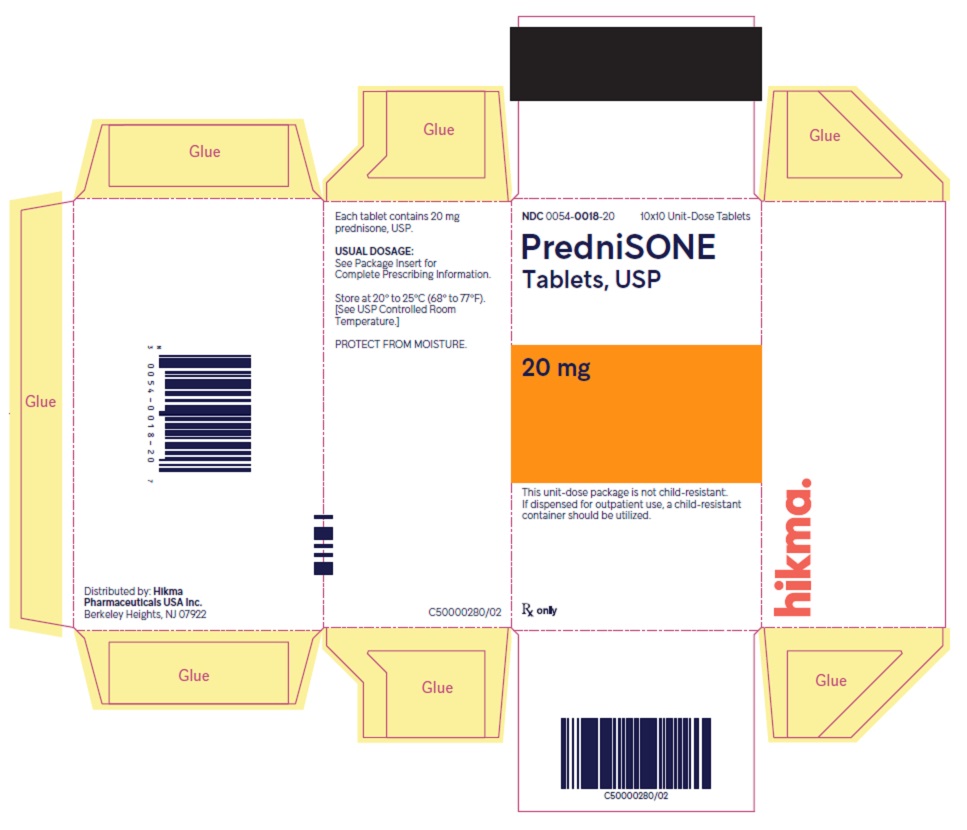
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0018-20 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 20 mg
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0018-25 100 Tablets - PredniSONE Tablets, USP - 20 mg
-
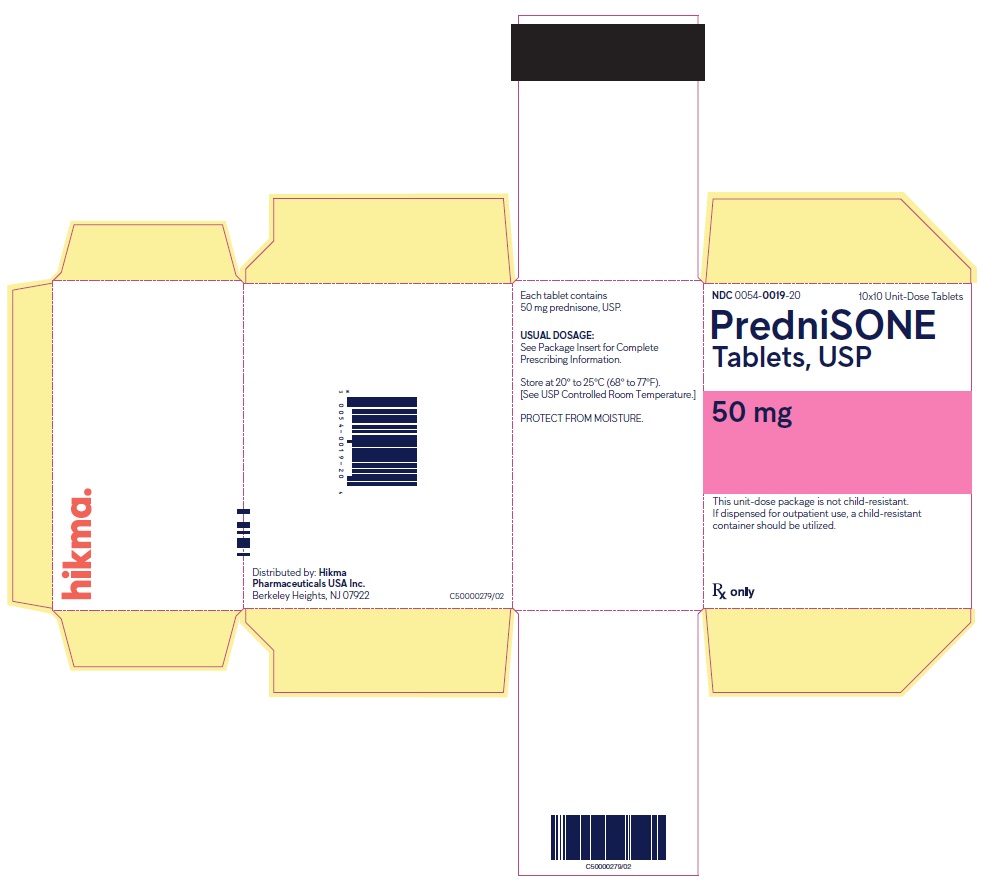
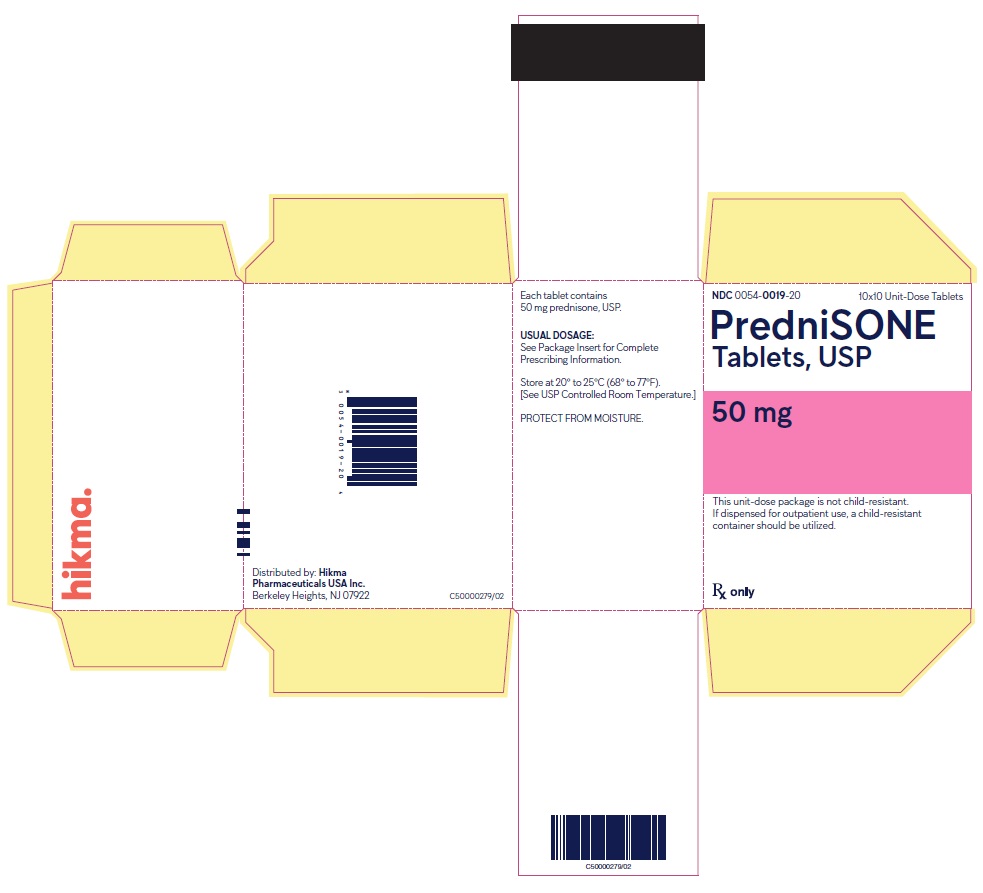
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0019-20 10x10 Unit-Dose Tablets - PredniSONE Tablets, USP - 50 mg
-
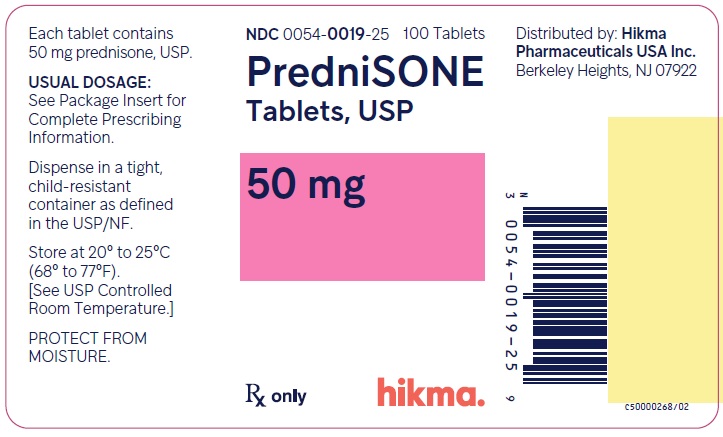
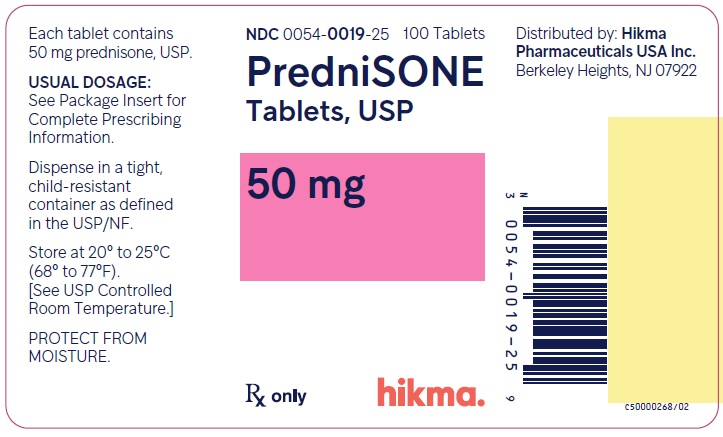
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-0019-25 100 Tablets - PredniSONE Tablets, USP - 50 mg
-
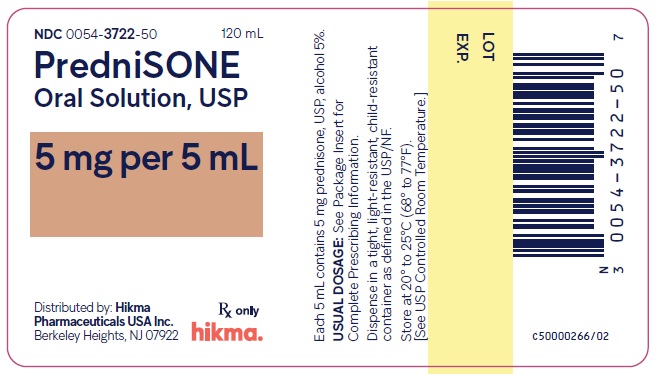
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-3722-50 120 mL - PredniSONE Oral Solution, USP - 5 mg per 5 mL
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0054-3721-44 30 mL - PredniSONE Intensol™ Oral Solution (Concentrate) 5 mg per mL
-
INGREDIENTS AND APPEARANCEProduct Information