Label: LUMIGAN- bimatoprost solution/ drops
- NDC Code(s): 0023-3205-02, 0023-3205-03, 0023-3205-05, 0023-3205-08
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUMIGAN 0.01% safely and effectively. See full prescribing information for LUMIGAN 0.01%. LUMIGAN® (bimatoprost ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension.
-
2
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. LUMIGAN® (bimatoprost ophthalmic solution) 0.01% should not be administered more than once daily since it has ...
-
3
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing bimatoprost 0.1 mg/mL.
-
4
CONTRAINDICATIONS
LUMIGAN® 0.01% is contraindicated in patients with hypersensitivity to bimatoprost or to any of the ingredients [see Adverse Reactions (6.2)].
-
5
WARNINGS AND PRECAUTIONS
5.1 - Pigmentation - Bimatoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the ...
-
6
ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling: Pigmentation including blepharal pigmentation and iris hyperpigmentation [see Warnings and Precautions (5.1)] Eyelash ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies of LUMIGAN® (bimatoprost ophthalmic solution) 0.01% administration in pregnant women. There is no ...
-
10
OVERDOSAGE
No information is available on overdosage in humans. If overdose with LUMIGAN® (bimatoprost ophthalmic solution) 0.01% occurs, treatment should be symptomatic. In oral (by gavage) mouse and rat ...
-
11
DESCRIPTION
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Bimatoprost, a prostaglandin analog, is a synthetic structural analog of prostaglandin with ocular hypotensive activity. It selectively mimics the effects of ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Bimatoprost was not carcinogenic in either mice or rats when administered by oral gavage for 104 weeks at ...
-
14
CLINICAL STUDIES
In a 12-month clinical study of patients with open angle glaucoma or ocular hypertension with an average baseline IOP of 23.5 mmHg, the IOP-lowering effect of LUMIGAN® 0.01% once daily (in the ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is supplied sterile in opaque white low density polyethylene ophthalmic dispenser bottles and tips with turquoise polystyrene caps in the ...
-
17
PATIENT COUNSELING INFORMATION
Potential for Pigmentation - Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Also inform patients about the possibility of eyelid skin ...
-
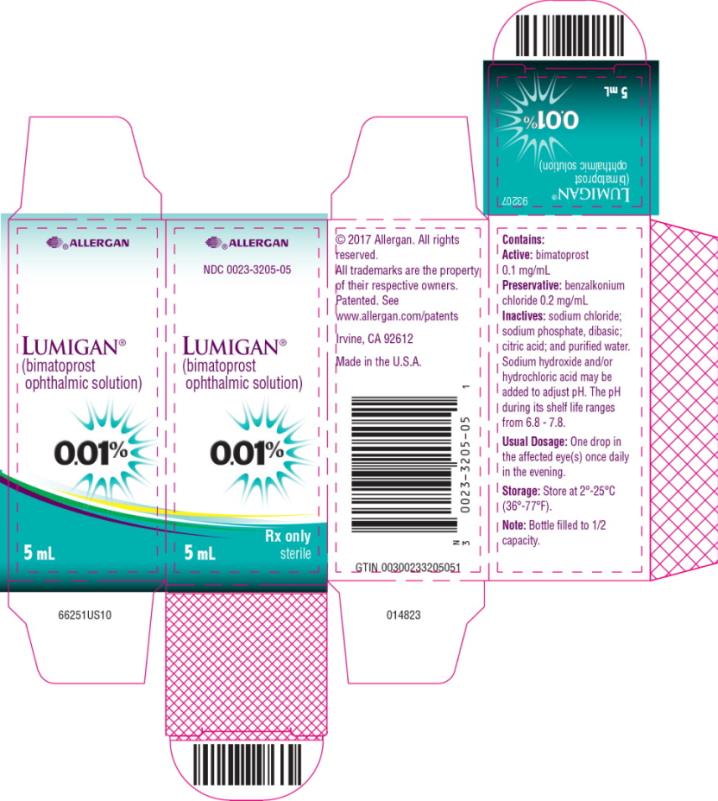
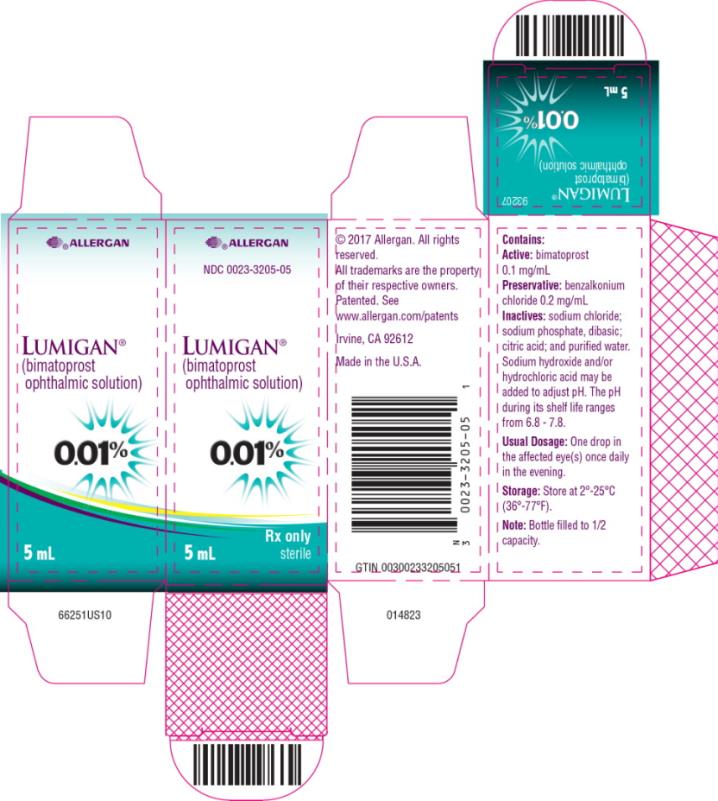
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - abbvie - NDC 0023-3205-05 - LUMIGAN® (bimatoprost - ophthalmic solution) 0.01% Rx only - 5 mL sterile
-
INGREDIENTS AND APPEARANCEProduct Information