Label: XALATAN- latanoprost solution
- NDC Code(s): 0013-8303-04
- Packager: PFIZER LABORATORIES DIV PFIZER INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XALATAN safely and effectively. See full prescribing information for XALATAN. XALATAN® (latanoprost ophthalmic solution) 0.005% ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE XALATAN is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
-
2 DOSAGE AND ADMINISTRATION The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal. The dosage of XALATAN should not ...
-
3 DOSAGE FORMS AND STRENGTHS Ophthalmic solution containing latanoprost 50 mcg/mL (0.005%).
-
4 CONTRAINDICATIONS Known hypersensitivity to latanoprost, benzalkonium chloride, or any other ingredients in this product.
-
5 WARNINGS AND PRECAUTIONS 5.1 Pigmentation - XALATAN has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue ...
-
6 ADVERSE REACTIONS The following adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the label: • Iris pigmentation changes [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of XALATAN administration in pregnant women.to inform drug-associated risks. In animal reproduction studies ...
-
10 OVERDOSAGE IV infusion of up to 3 mcg/kg of latanoprost in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment with XALATAN and no adverse reactions were ...
-
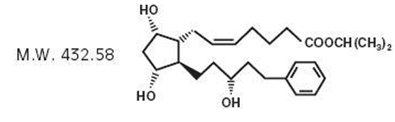
11 DESCRIPTION Latanoprost is a prostaglandin F2α analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Latanoprost is a prostaglandin F2α analogue that is believed to reduce the IOP by increasing the outflow of aqueous humor. Studies in animals and man suggest that the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Latanoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses of up to 170 ...
-
14 CLINICAL STUDIES 14.1 Elevated Baseline IOP - Patients with mean baseline IOP of 24 – 25 mmHg who were treated for 6 months in multi-center, randomized, controlled trials demonstrated 6 – 8 mmHg reductions in ...
-
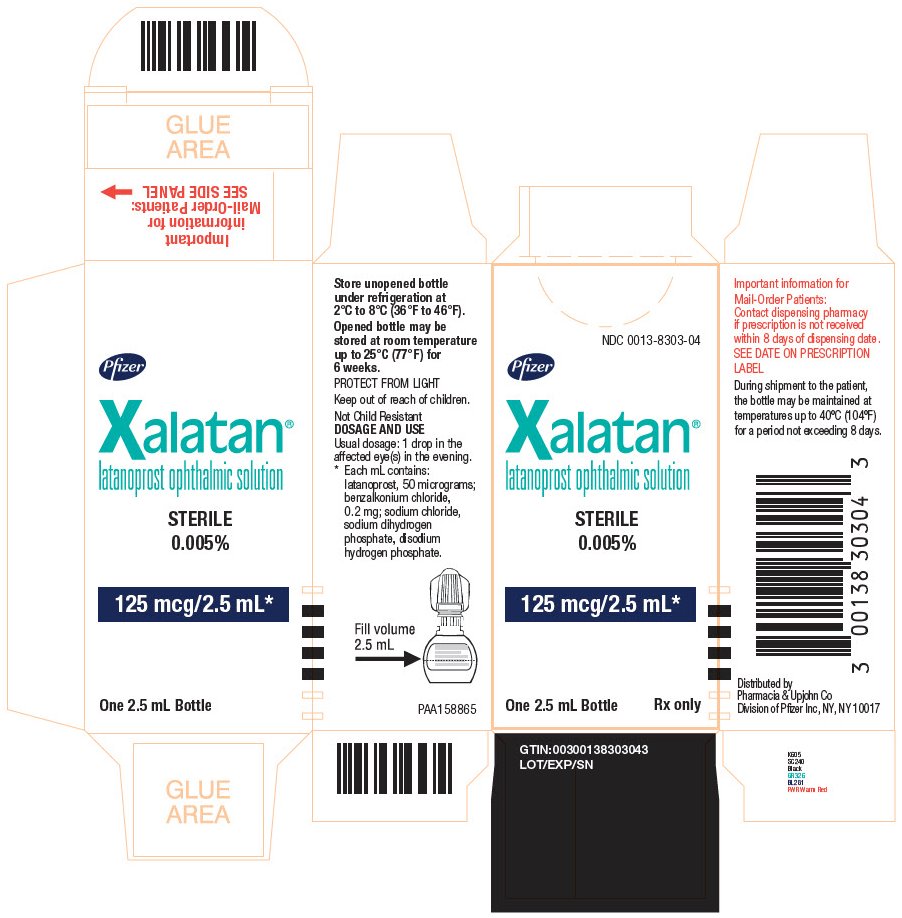
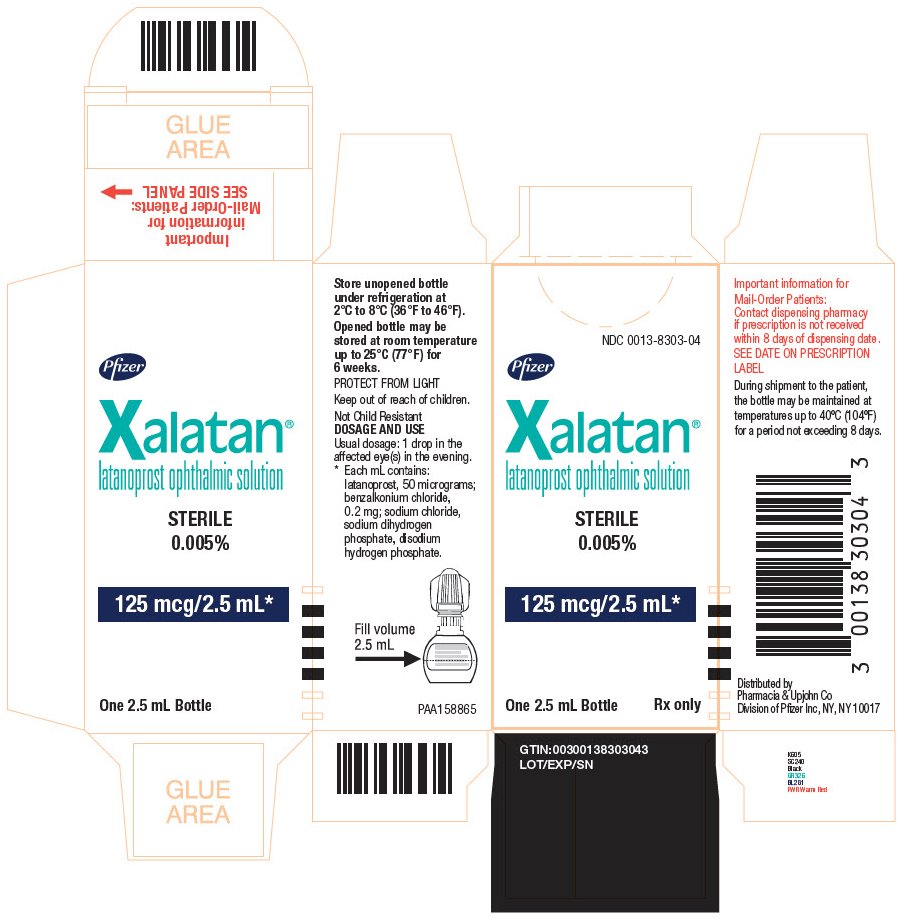
16 HOW SUPPLIED/STORAGE AND HANDLING XALATAN is a clear, isotonic, buffered, preserved colorless solution of latanoprost 50 mcg/mL (0.005%). It is supplied as a 2.5 mL solution in a 5 mL clear low density polyethylene bottle with a ...
-
17 PATIENT COUNSELING INFORMATION Potential for Pigmentation - Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin ...
-
PRINCIPAL DISPLAY PANEL - 2.5 mL NDC 0013-8303-04 - Pfizer - Xalatan® latanoprost ophthalmic solution - STERILE - 0.005% 125 mcg/2.5 mL* One 2.5 mL Bottle Rx only - Store unopened bottle - under refrigeration at - 2°C to 8°C ...
-
INGREDIENTS AND APPEARANCEProduct Information