Label: HUMULIN 70/30- insulin human injection, suspension
HUMULIN 70/30 KWIKPEN- insulin human injection, suspension
- NDC Code(s): 0002-8715-01, 0002-8715-17, 0002-8803-01, 0002-8803-59
- Packager: Eli Lilly and Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HUMULIN 70/30 safely and effectively. See full prescribing information for HUMULIN 70/30. HUMULIN 70/30 (insulin isophane human and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
HUMULIN 70/30 is a fixed ratio insulin formulation indicated to improve glycemic control in adults with diabetes mellitus.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions - Inspect HUMULIN 70/30 visually before use. It should not contain particulate matter and should appear uniformly cloudy after mixing. Do not use ...
-
3 DOSAGE FORMS AND STRENGTHS
Injectable suspension: 70% insulin isophane human and 30% insulin human, 100 units/mL (U-100), white and cloudy suspension available as: 10 mL multiple-dose vial - 3 mL multiple-dose vial - 3 mL ...
-
4 CONTRAINDICATIONS
HUMULIN 70/30 is contraindicated: During episodes of hypoglycemia [see Warnings and Precautions (5.3)], and - In patients who have had hypersensitivity reactions to HUMULIN 70/30 or any of its ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a HUMULIN 70/30 KwikPen or Syringe Between Patients - HUMULIN 70/30 KwikPens must never be shared between patients, even if the needle is changed. Patients using HUMULIN 70/30 ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions (5.3)]. Hypoglycemia Due to Medication Errors [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS
Table 1: Clinically Significant Drug Interactions with HUMULIN 70/30 - Drugs that May Increase the Risk of Hypoglycemia - Drugs:Antidiabetic agents, ACE inhibitors, angiotensin II receptor ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Available data from published studies over decades have not established an association with human insulin use during pregnancy and major birth defects ...
-
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6)]. Mild episodes of hypoglycemia can be treated with oral glucose. Adjustments in drug ...
-
11 DESCRIPTION
Insulin human is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli. The amino acid sequence of insulin human is identical to human insulin and ...
-
12 CLINICAL PHARMACOLOGY
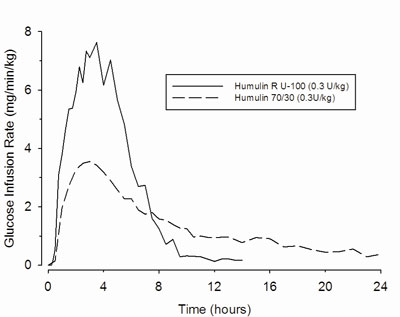
12.1 Mechanism of Action - HUMULIN 70/30 lowers blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity and fertility studies were not performed in animals. Biosynthetic human insulin was not genotoxic in the in vivo ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - HUMULIN 70/30 (insulin isophane human and insulin human) injectable suspension is 70% insulin isophane human and 30% insulin human, 100 units/mL (U-100), a white and cloudy ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Never Share a HUMULIN 70/30 KwikPen or Syringe Between Patients - Advise patients ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - HUMULIN® (HU-mu-lin) 70/30 - (insulin isophane human and insulin human) injectable suspension, for subcutaneous use - Do not share your HUMULIN 70/30 KwikPen or syringes with ...
-
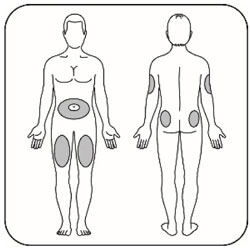
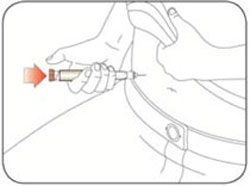
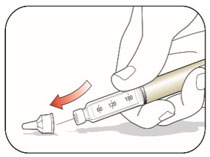
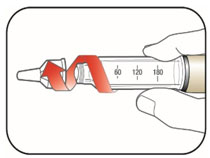
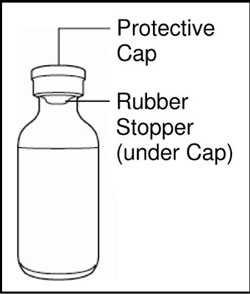
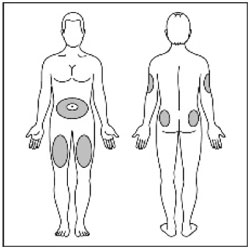
VIAL INSTRUCTIONS FOR USEInstructions for Use - HUMULIN® (HU-mu-lin) 70/30 - (insulin isophane human and insulin human) injectable suspension, for subcutaneous use - 3 mL or 10 mL multiple-dose vial (100 units/mL) Read the ...
-
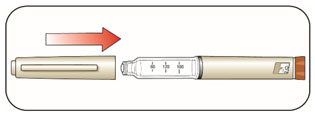
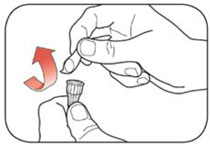
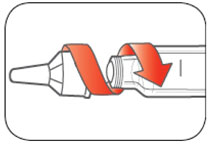
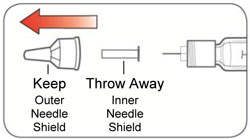
KWIKPEN INSTRUCTIONS FOR USEInstructions for Use - HUMULIN® 70/30 KwikPen® (insulin isophane human and insulin human) injectable suspension, for subcutaneous use - 3 mL single-patient-use pen (100 units/mL) Read the ...
-
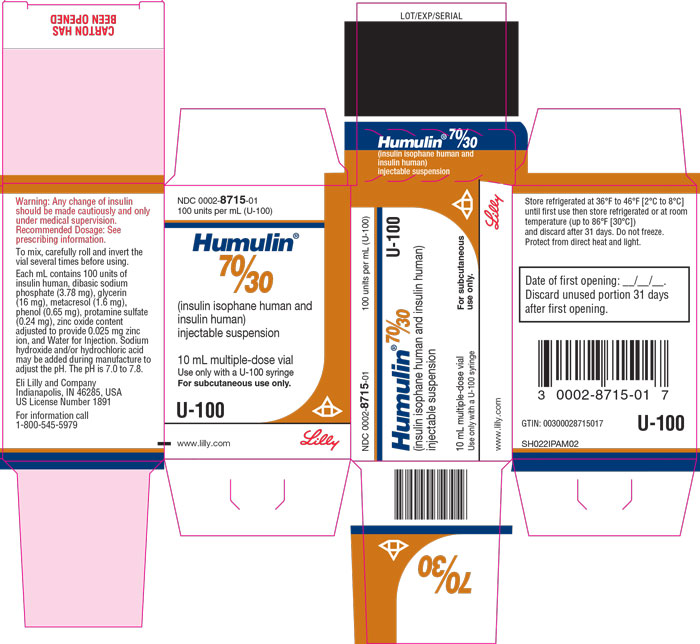
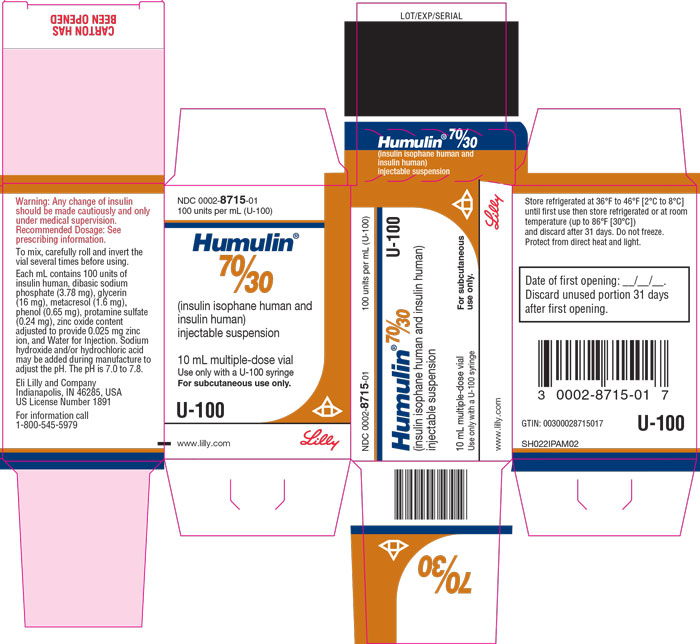
PACKAGE CARTON – HUMULIN 70/30 Vial 10 mL NDC 0002-8715-01 - 100 units per mL (U-100) Humulin® 70/30 - (insulin isophane human and insulin human) injectable suspension - 10 mL multiple-dose vial - Use only with a U-100 syringe - For ...
-
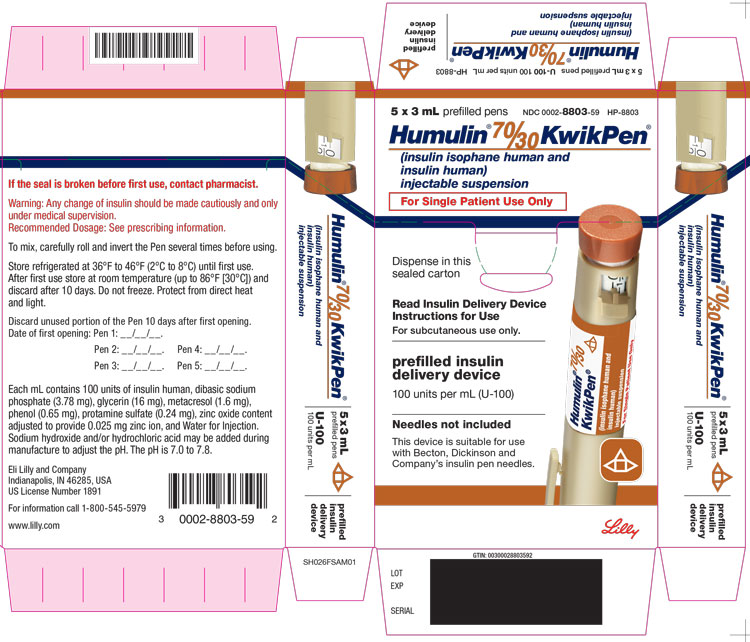
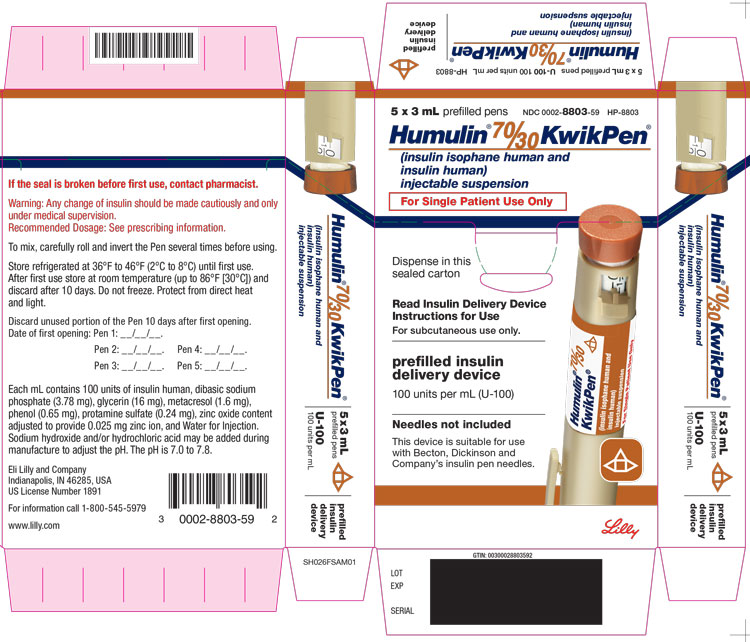
PACKAGE LABEL – Humulin 7030 KwikPen 3mL5 x 3 mL prefilled pens - NDC 0002-8803-59 - HP-8803 - Humulin® 70/30 KwikPen® (insulin isophane human and insulin human) injectable suspension For Single Patient Use Only - Dispense in this ...
-
INGREDIENTS AND APPEARANCEProduct Information

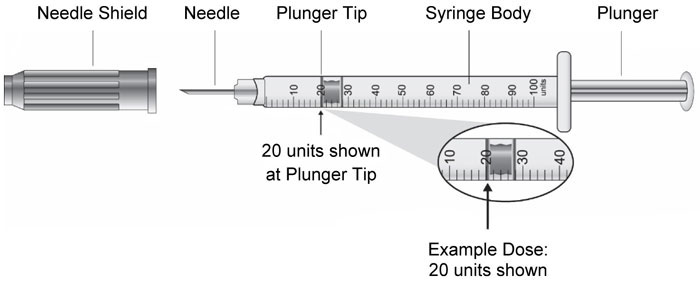
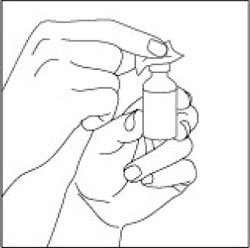
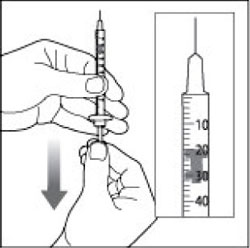 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)
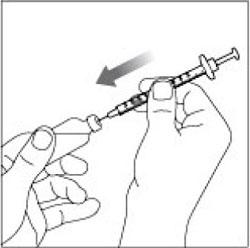
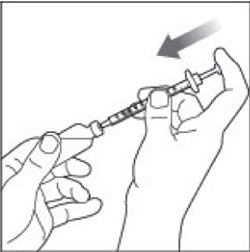
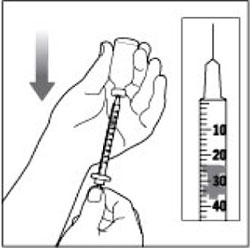 (Example Dose: 20 units Plunger is shown at 24 units)
(Example Dose: 20 units Plunger is shown at 24 units)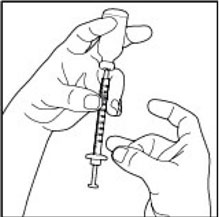
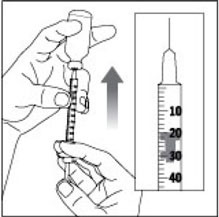 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)
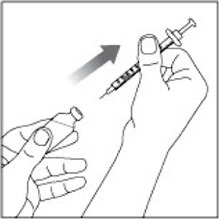
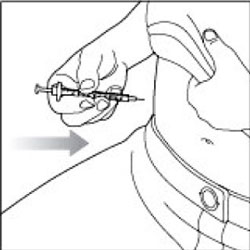
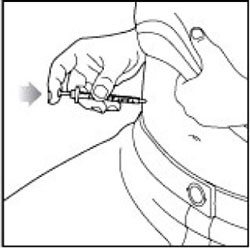
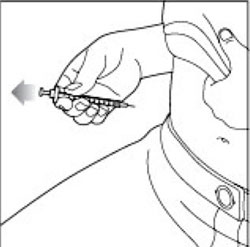


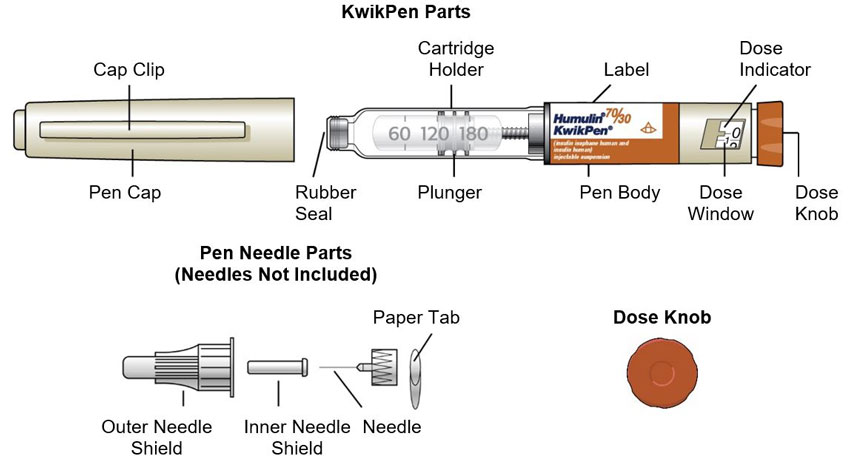
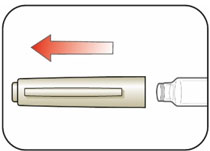
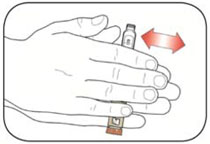
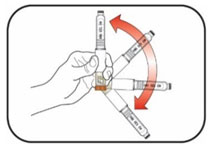
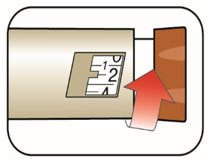
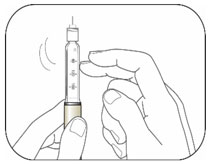
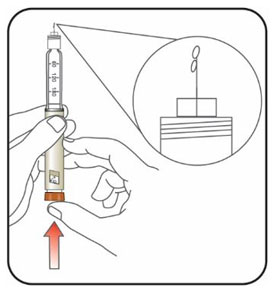
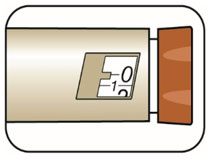
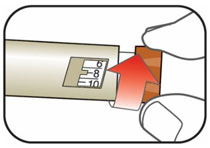
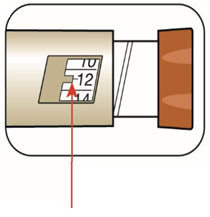 (Example: 12 units shown in the Dose Window)
(Example: 12 units shown in the Dose Window)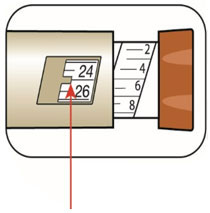 (Example: 25 units shown in the Dose Window)
(Example: 25 units shown in the Dose Window)