Label: BALFAXAR (prothrombin complex concentrate- human powder, for solution
- NDC Code(s): 68982-261-01, 68982-261-02, 68982-261-81, 68982-261-82
- Packager: Octapharma USA Inc
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BALFAXAR safely and effectively. See full prescribing information for BALFAXAR. BALFAXAR (prothrombin complex concentrate ...
-
Table of ContentsTable of Contents
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonist (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic event. Resumption of anticoagulation should be carefully considered as soon as the risk of thromboembolic events outweighs the risk of acute bleeding.
- Both fatal and non-fatal arterial and venous thromboembolic complications have been reported with BALFAXAR in clinical trials and post marketing surveillance. Monitor patients receiving BALFAXAR for signs and symptoms of thromboembolic events. [see Warnings and Precautions ( 5.2 )].
- BALFAXAR may not be suitable in patients with thromboembolic events in the prior 3 months. [see Warnings and Precautions ( 5.2 )].
Close -
1 INDICATIONS AND USAGEBALFAXAR (prothrombin complex concentrate, human-lans) is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by ...
-
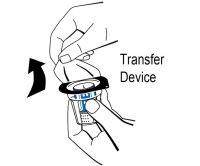
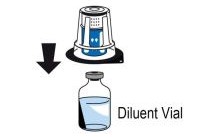
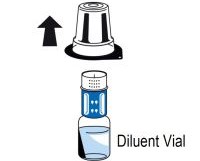
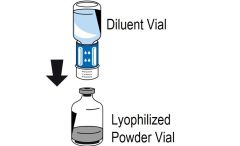
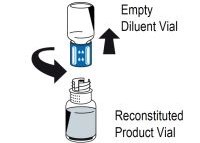
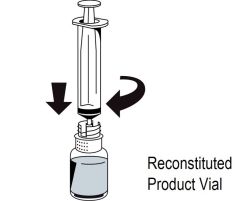
2 DOSAGE AND ADMINISTRATIONFor intravenous use after reconstitution only - . 2.1 Dosage - Measurement of INR prior to treatment and close to the time of dosing is important because coagulation factors may be unstable ...
-
3 DOSAGE FORMS AND STRENGTHSBALFAXAR is a sterile, white to ice-blue lyophilized powder for reconstitution for intravenous use. It is provided in a single-dose vial with a nominal strength of 500 Factor IX units in 20 mL ...
-
4 CONTRAINDICATIONSKnown anaphylactic or severe systemic reactions to BALFAXAR or any of the components of the product. For a complete listing of ingredients, [ see - Description - ( 11 - ) ]. Known allergy to ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - If severe allergic or anaphylactic-type reactions occur, immediately discontinue administration of BALFAXAR and initiate appropriate treatment. Therapeutic ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with BALFAXAR use in pregnancy to inform on drug-associated risk. Animal reproduction studies have not been conducted with BALFAXAR. It is not ...
-
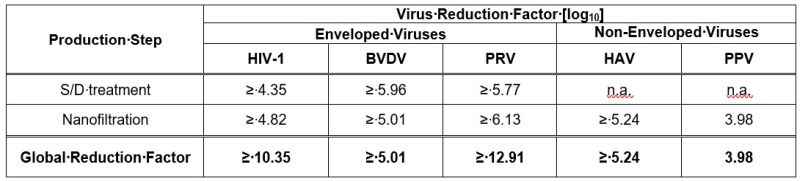
11 DESCRIPTIONBALFAXAR is a human plasma-derived, purified, virus inactivated and nanofiltered non-activated Prothrombin Complex Concentrate (PCC) containing the coagulation factors II, VII, IX, and X and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The administration of BALFAXAR provides a rapid increase in plasma levels of the vitamin K-dependent coagulation factors (FII, FVII, FIX, FX) and antithrombotic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate the carcinogenic potential of BALFAXAR or studies to determine the effects of BALFAXAR on ...
-
14 CLINICAL STUDIESThe efficacy of BALFAXAR was assessed in a randomized, double-blind, multicenter study in comparison to Kcentra, for the reversal of vitamin K antagonist induced anticoagulation in patients ...
-
15 REFERENCESKoehler M: Thrombogenicity of prothrombin complex concentrates. Thrombosis Research 1999;95:S13-S17. Warkentin TE, Crowther MA: Reversing anticoagulants both old and new. Can.J. Anaesth ...
-
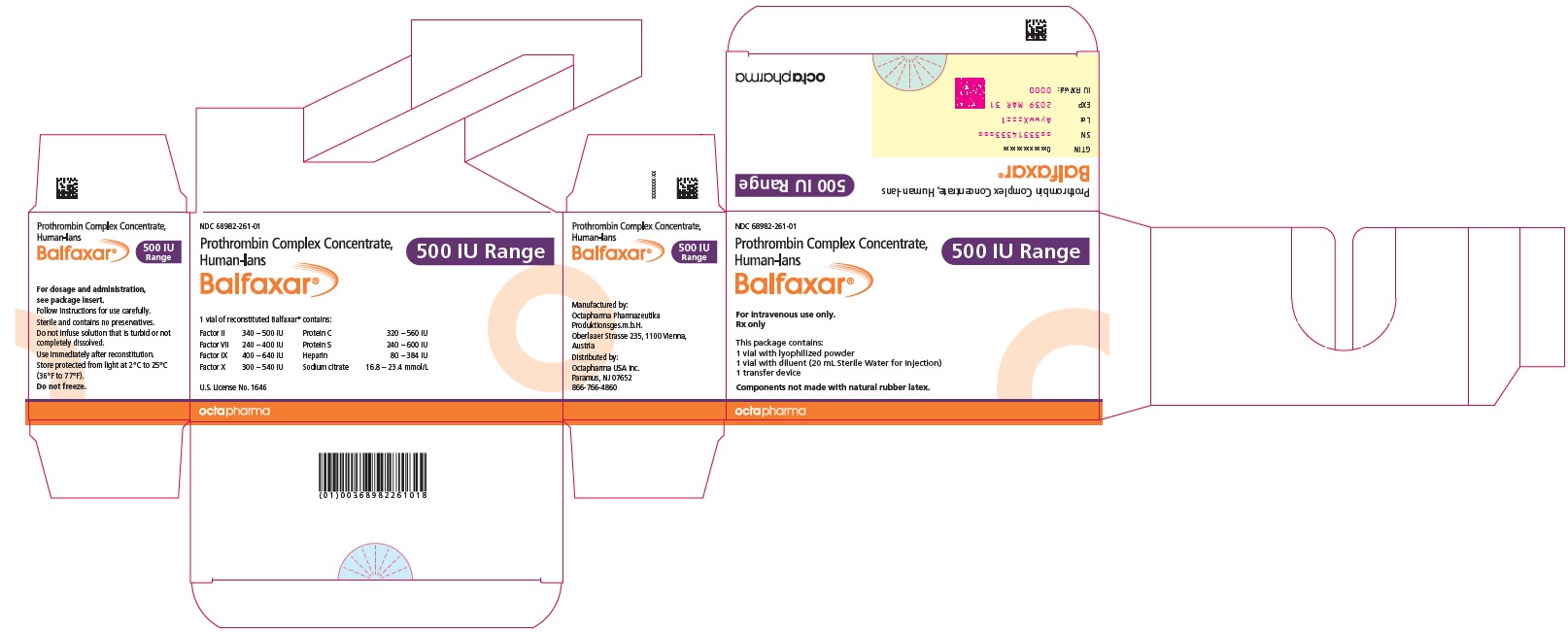
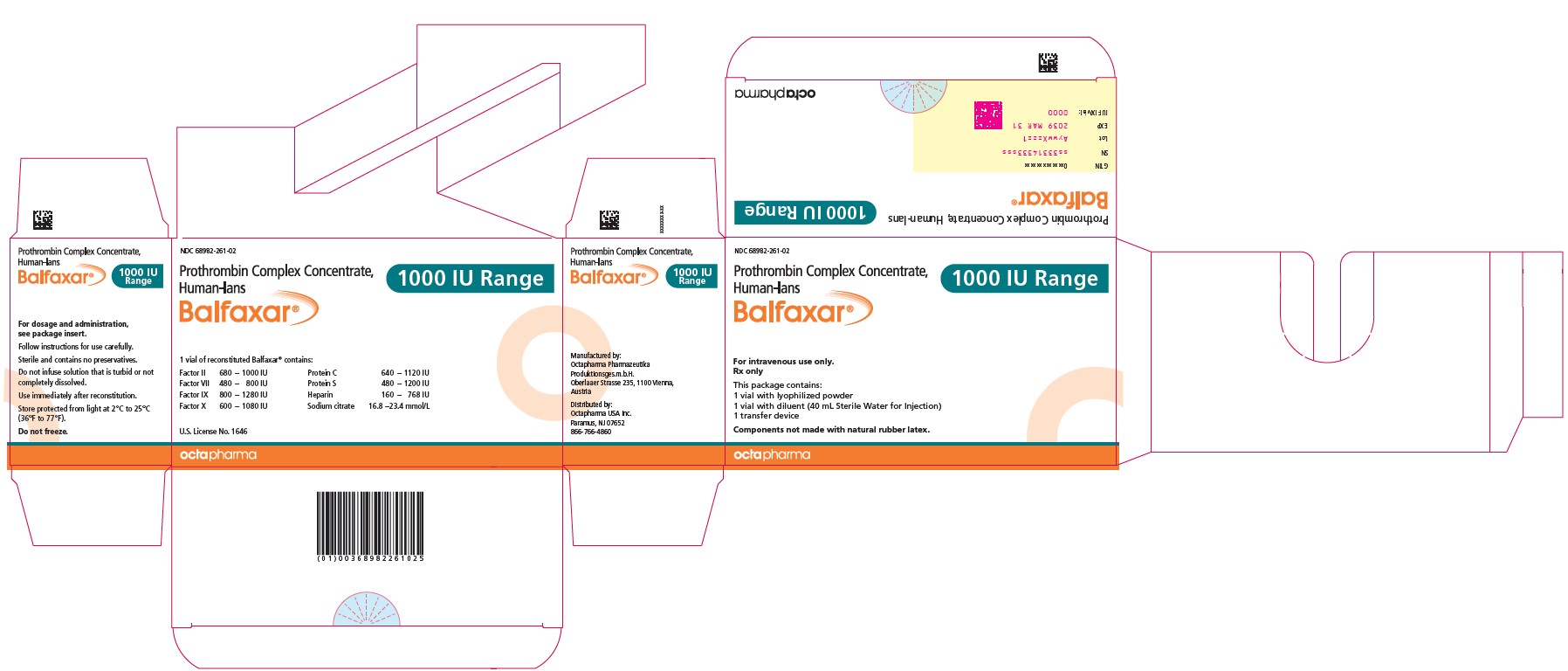
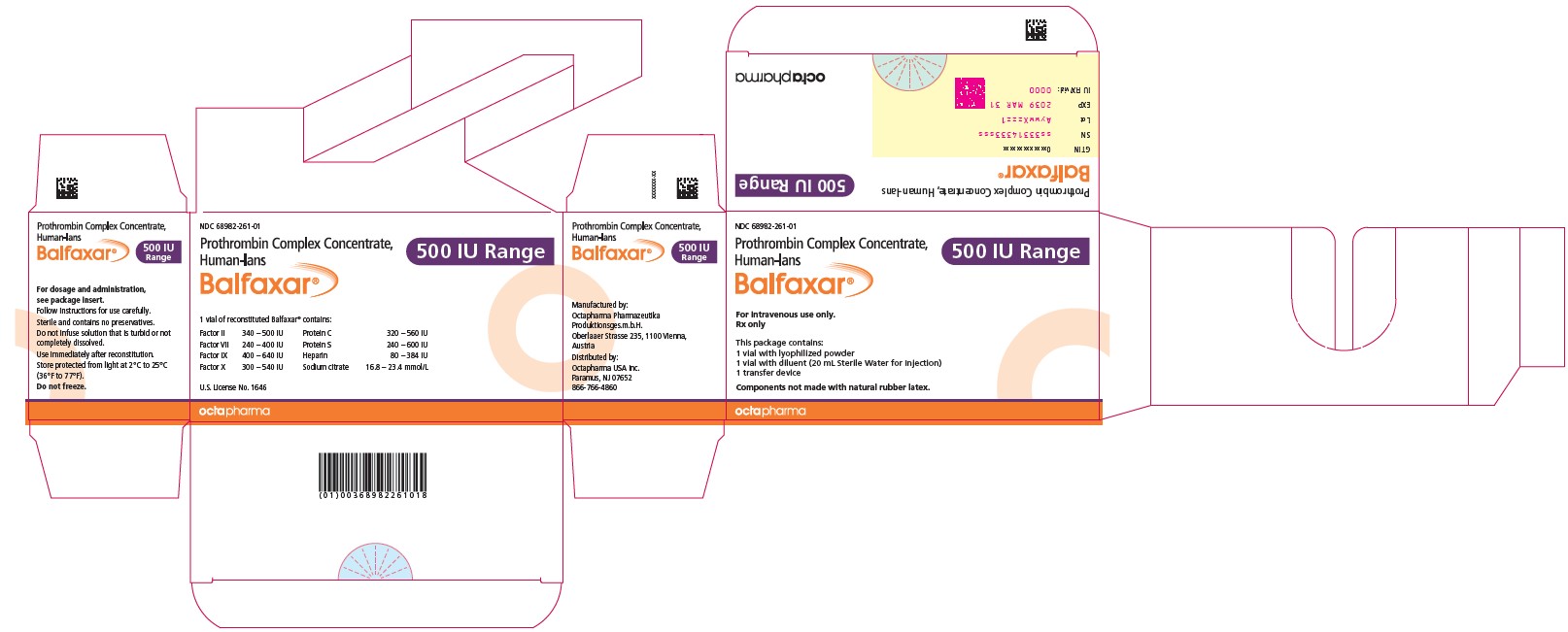
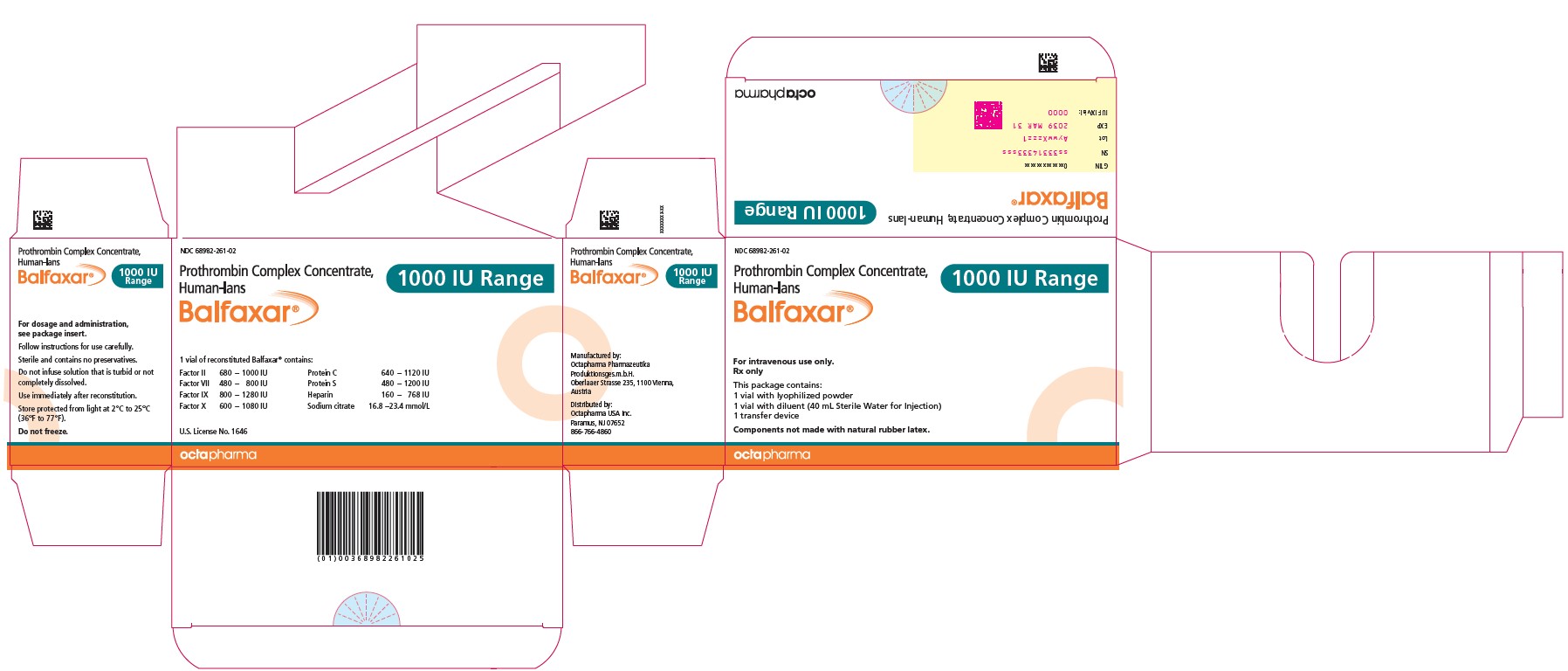
16 HOW SUPPLIED/STORAGE AND HANDLINGBALFAXAR is supplied in single-dose vials. Carton - NDC Number - Container (Vial) NDC Number - Size - Color coding - 68982-261-01 - 68982-261-02 - 68982-261-81 - 68982-261-82 - 500 IU Range FIX ...
-
17 PATIENT COUNSELING INFORMATIONInform patients of the signs and symptoms of allergic hypersensitivity reactions, such as urticaria, rash, tightness of the chest, wheezing, hypotension and/or anaphylaxis experienced during or ...
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANELProthrombin Complex Concentrate (Human) BALFAXAR - 500 IU Range FIX in 20 mL - NDC 68982-261-01 - BALFAXAR - 1000 IU Range FIX in 40 mL - NDC 68982-261-01
-
INGREDIENTS AND APPEARANCEProduct Information