Label: BETASERON- interferon beta-1b kit
-
NDC Code(s):
50419-524-01,
50419-524-05,
50419-524-09,
50419-524-35, view more50419-524-81
- Packager: Bayer HealthCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BETASERON safely and effectively. See full prescribing information for BETASERON. BETASERON (interferon beta-1b) for injection ...These highlights do not include all the information needed to use BETASERON safely and effectively. See full prescribing information for BETASERON.
BETASERON (interferon beta-1b) for injection, for subcutaneous use
Initial U.S. Approval: 1993RECENT MAJOR CHANGES
Warnings and Precautions (5.8) 7/2023
INDICATIONS AND USAGE
BETASERON is an interferon beta indicated for the treatment of relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
For injection: 0.3 mg of lyophilized powder in a single-dose vial for reconstitution (3)
CONTRAINDICATIONS
History of hypersensitivity to natural or recombinant interferon beta, albumin or mannitol (4)
WARNINGS AND PRECAUTIONS
- •
- Hepatic Injury: Monitor liver function tests and signs and symptoms of hepatic injury; consider discontinuing BETASERON if serious hepatic injury occurs. (5.1, 5.11)
- •
- Anaphylaxis and Other Allergic Reactions: Discontinue if anaphylaxis occurs. (5.2)
- •
- Depression and Suicide: Advise patients to immediately report any symptom of depression and/or suicidal ideation; consider discontinuation of BETASERON if depression occurs. (5.3)
- •
- Congestive Heart Failure (CHF): Monitor patients with CHF for worsening of cardiac symptoms; consider discontinuation of BETASERON if worsening of CHF occurs. (5.4)
- •
- Injection Site Reactions Including Necrosis: Do not administer BETASERON into affected area until fully healed; if multiple lesions occur, change injection site or discontinue BETASERON until healing of skin lesions. (5.5)
- •
- Leukopenia: Monitor complete blood count. (5.6, 5.12)
- •
- Thrombotic Microangiopathy: Cases of thrombotic microangiopathy (TMA) have been reported. Discontinue BETASERON if clinical symptoms and laboratory findings consistent with TMA occur and a relationship to BETASERON is suspected. (5.7)
- •
- Pulmonary Arterial Hypertension: Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including BETASERON. Discontinue BETASERON if PAH is diagnosed. (5.8)
- •
- Flu-like Symptom Complex: Consider analgesics and/or antipyretics on injection days. (5.9)
- •
- Drug-induced Lupus Erythematosus: Cases of drug-induced lupus erythematosus have been reported. Discontinue BETASERON if patients develop new characteristic signs and symptoms. (5.11)
ADVERSE REACTIONS
In controlled clinical trials, the most common adverse reactions (at least 5% more frequent on BETASERON than on placebo) were: injection site reaction, lymphopenia, flu-like symptoms, myalgia, leukopenia, neutropenia, increased liver enzymes, headache, hypertonia, pain, rash, insomnia, abdominal pain, and asthenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Reconstitution of the Lyophilized Powder
2.3 Important Administration Instructions
2.4 Premedication for Flu-like Symptoms
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Injury
5.2 Anaphylaxis and Other Allergic Reactions
5.3 Depression and Suicide
5.4 Congestive Heart Failure
5.5 Injection Site Reactions Including Necrosis
5.6 Leukopenia
5.7 Thrombotic Microangiopathy
5.8 Pulmonary Arterial Hypertension
5.9 Flu-like Symptom Complex
5.10 Seizures
5.11 Drug-induced Lupus Erythematosus
5.12 Monitoring for Laboratory Abnormalities
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Stability and Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEBETASERON is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive ...
BETASERON is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended starting dose is 0.0625 mg (0.25 mL) subcutaneously every other day, with dose increases over a six-week period to the recommended dose of 0.25 mg (1 mL ...
2.1 Dosing Information
The recommended starting dose is 0.0625 mg (0.25 mL) subcutaneously every other day, with dose increases over a six-week period to the recommended dose of 0.25 mg (1 mL) every other day (see Table 1).
Table 1: Schedule for Dose Titration - *
- Dosed every other day, subcutaneously
BETASERON
Dose*
Percentage of
recommended dose
Volume
Weeks 1-2
0.0625 mg
25%
0.25 mL
Weeks 3-4
0.125 mg
50%
0.5 mL
Weeks 5-6
0.1875 mg
75%
0.75 mL
Week 7 and thereafter
0.25 mg
100%
1 mL
If a dose of BETASERON is missed, then it should be taken as soon as the patient remembers or is able to take it. The patient should not take BETASERON on two consecutive days. The next injection should be taken about 48 hours (two days) after that dose. If the patient accidentally takes more than their prescribed dose, or takes it on two consecutive days, they should be instructed to call their healthcare provider immediately.
2.2 Reconstitution of the Lyophilized Powder
(a) Prior to reconstitution, verify that the vial containing lyophilized BETASERON is not cracked or damaged. Do not use cracked or damaged vials.
(b) To reconstitute lyophilized BETASERON for injection, attach the pre-filled syringe containing the diluent (Sodium Chloride, 0.54% Solution) to the BETASERON vial using the vial adapter.
(c) Slowly inject 1.2 mL of diluent into the BETASERON vial.
(d) Gently swirl the vial to dissolve the lyophilized powder completely; do not shake. Foaming may occur during reconstitution or if the vial is swirled or shaken too vigorously. If foaming occurs, allow the vial to sit undisturbed until the foam settles.
(e) 1 mL of reconstituted BETASERON solution contains 0.25 mg of interferon beta-1b.
(f) After reconstitution, if not used immediately, refrigerate the reconstituted BETASERON solution at 35°F to 46°F (2°C to 8°C) and use within three hours. Do not freeze.
2.3 Important Administration Instructions
(a) BETASERON is intended for use under the guidance and supervision of a physician. If patients or caregivers are to administer BETASERON, train them in the proper technique for self‐administering subcutaneous injections using the prefilled syringe or the optional injection device. The BETACONNECT autoinjector has three adjustable injection depth settings; the healthcare provider should determine the proper depth setting and injection technique. Use only the syringes in the BETASERON packaging with the BETACONNECT autoinjector.
The initial BETASERON injection should be performed under the supervision of an appropriately qualified healthcare provider. Users should demonstrate competency in all aspects of the BETASERON injection prior to independent use. If a patient is to self‐administer BETASERON, the physical and cognitive ability of that patient to self‐administer and properly dispose of syringes should be assessed. Patients with severe neurological deficits should not self‐administer injections without assistance from a trained caregiver.
Appropriate instruction for self‐injection or injection by another person should be provided to the patient or their caregiver, including careful review of the BETASERON Medication Guide, the prefilled syringe Instructions for Use, and the BETACONNECT autoinjector Instructions for Use that accompanies the product.
(b) Visually inspect the reconstituted BETASERON solution before use; discard if it contains particulate matter or is discolored.
(c) Keeping the syringe and vial adapter in place, turn the assembly over so that the vial is on top. Withdraw the appropriate dose of BETASERON solution. Remove the vial from the vial adapter before injecting BETASERON.
(d) Use safe disposal procedures for needles and syringes.
(e) Do not re-use needles or syringes.
(f) Advise patients and caregivers to rotate sites for subcutaneous injections to minimize the likelihood of severe injection site reactions, including necrosis or localized infection [see Warnings and Precautions (5.5)].
Close2.4 Premedication for Flu-like Symptoms
Concurrent use of analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms associated with BETASERON use [see Warnings and Precautions (5.8)].
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 0.3 mg lyophilized powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSBETASERON is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta, Albumin (Human), or any other component of the formulation.
BETASERON is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta, Albumin (Human), or any other component of the formulation.
Close -
5 WARNINGS AND PRECAUTIONS5.1 Hepatic Injury - Severe hepatic injury including cases of hepatic failure, some of which have been due to autoimmune hepatitis, has been rarely reported in patients taking BETASERON. In some ...
5.1 Hepatic Injury
Severe hepatic injury including cases of hepatic failure, some of which have been due to autoimmune hepatitis, has been rarely reported in patients taking BETASERON. In some cases, these events have occurred in the presence of other drugs or comorbid medical conditions that have been associated with hepatic injury. Consider the potential risk of BETASERON used in combination with known hepatotoxic drugs or other products (eg, alcohol) prior to BETASERON administration, or when adding new agents to the regimen of patients already on BETASERON. Monitor patients for signs and symptoms of hepatic injury. Consider discontinuing BETASERON if serum transaminase levels significantly increase, or if they are associated with clinical symptoms such as jaundice.
Asymptomatic elevation of serum transaminases is common in patients treated with BETASERON. In controlled clinical trials, elevations of SGPT to greater than five times baseline value were reported in 12% of patients receiving BETASERON (compared to 4% on placebo), and increases of SGOT to greater than five times baseline value were reported in 4% of patients receiving BETASERON (compared to 1% on placebo), leading to dose-reduction or discontinuation of treatment in some patients [see Adverse Reactions (6.1)]. Monitor liver function tests [see Warnings and Precautions (5.12)].
5.2 Anaphylaxis and Other Allergic Reactions
Anaphylaxis has been reported as a rare complication of BETASERON use. Other allergic reactions have included dyspnea, bronchospasm, tongue edema, skin rash and urticaria [see Adverse Reactions (6.1)]. Discontinue BETASERON if anaphylaxis occurs.
5.3 Depression and Suicide
Depression and suicide have been reported to occur with increased frequency in patients receiving interferon beta products, including BETASERON. Advise patients to report any symptom of depression and/or suicidal ideation to their healthcare provider. If a patient develops depression, discontinuation of BETASERON therapy should be considered.
In randomized controlled clinical trials, there were three suicides and eight suicide attempts among the 1532 patients on BETASERON compared to one suicide and four suicide attempts among 965 patients on placebo.
5.4 Congestive Heart Failure
Monitor patients with pre-existing congestive heart failure (CHF) for worsening of their cardiac condition during initiation of and continued treatment with BETASERON. While beta interferons do not have any known direct-acting cardiac toxicity, cases of CHF, cardiomyopathy, and cardiomyopathy with CHF have been reported in patients without known predisposition to these events, and without other known etiologies being established. In some cases, these events have been temporally related to the administration of BETASERON. Recurrence upon rechallenge was observed in some patients. Consider discontinuation of BETASERON if worsening of CHF occurs with no other etiology.
5.5 Injection Site Reactions Including Necrosis
Injection site reactions, including injection site necrosis, can occur with the use of interferon beta products, including BETASERON. Injection site necrosis (ISN) was reported in 4% of BETASERON-treated patients in controlled clinical trials (compared to 0% on placebo) [see Adverse Reactions (6.1)]. Typically, ISN occurs within the first four months of therapy, although postmarketing reports have been received of ISN occurring over one year after initiation of therapy. The necrotic lesions are typically 3 cm or less in diameter, but larger areas have been reported. Generally the necrosis has extended only to subcutaneous fat, but has extended to the fascia overlying muscle. In some lesions where biopsy results are available, vasculitis has been reported. For some lesions, debridement, and/or skin grafting have been required. In most cases healing was associated with scarring.
In controlled clinical trials, injection site reactions occurred in 78% of patients receiving BETASERON with injection site necrosis in 4%. Injection site inflammation (42%), injection site pain (16%), injection site hypersensitivity (4%), injection site necrosis (4%), injection site mass (2%), injection site edema (2%), and nonspecific reactions were significantly associated with BETASERON treatment. The incidence of injection site reactions tended to decrease over time. Approximately 69% of patients experienced injection site reactions during the first three months of treatment, compared to approximately 40% at the end of the studies.
Injection site abscesses and cellulitis have been reported in the postmarketing setting with use of interferon beta products including BETASERON. Some cases required treatment with hospitalization for surgical drainage and intravenous antibiotics. Periodically evaluate patient understanding and use of aseptic self-injection techniques and procedures, particularly if injection site necrosis has occurred. Patients should be advised of the importance of rotating injection sites with each dose. Whether to discontinue therapy following a single site of necrosis is dependent on the extent of necrosis. For patients who continue therapy with BETASERON after injection site necrosis has occurred, avoid administration of BETASERON into the affected area until it is fully healed. If multiple lesions occur, change injection site or discontinue therapy until healing occurs.
5.6 Leukopenia
In controlled clinical trials, leukopenia was reported in 18% of patients receiving BETASERON (compared to 6% on placebo), leading to a reduction of the dose of BETASERON in some patients [see Adverse Reactions (6.1)]. Monitoring of complete blood and differential white blood cell counts is recommended. Patients with myelosuppression may require more intensive monitoring of complete blood cell counts, with differential and platelet counts.
5.7 Thrombotic Microangiopathy
Cases of thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, some fatal, have been reported with interferon beta products, including BETASERON.
Cases have been reported several weeks to years after starting interferon beta products. If clinical symptoms and laboratory findings consistent with TMA occur and a relationship to BETASERON is suspected, discontinue treatment and manage as clinically indicated.
5.8 Pulmonary Arterial Hypertension
Cases of pulmonary arterial hypertension (PAH) have been reported with interferon beta products, including BETASERON. PAH has occurred in patients treated with interferon beta products in the absence of other contributory factors. Many of the reported cases required hospitalization, including one case with interferon beta in which the patient underwent a lung transplant. PAH has developed at various time points after initiating therapy with interferon beta products and may occur several years after starting treatment.
Patients who develop unexplained symptoms (e.g., dyspnea, new or increasing fatigue) should be assessed for PAH. If alternative etiologies have been ruled out and a diagnosis of PAH is confirmed, discontinue treatment and manage as clinically indicated.
5.9 Flu-like Symptom Complex
In controlled clinical trials, the rate of flu-like symptom complex for patients on BETASERON was 57% [see Adverse Reactions (6.1)]. The incidence decreased over time, with 10% of patients reporting flu-like symptom complex at the end of the studies. The median duration of flu-like symptom complex in Study 1 was 7.5 days [see Clinical Studies (14)]. Analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms associated with BETASERON use.
5.10 Seizures
Seizures have been temporally associated with the use of beta interferons in clinical trials and postmarketing safety surveillance. It is not known whether these events were related to a primary seizure disorder, the effects of multiple sclerosis alone, the use of beta interferons, other potential precipitants of seizures (eg, fever), or to some combination of these.
5.11 Drug-induced Lupus Erythematosus
Cases of drug-induced lupus erythematosus have been reported with some interferon beta products, including BETASERON. Signs and symptoms of drug-induced lupus reported in BETASERON-treated patients have included rash, serositis, polyarthritis, nephritis, and Raynaud’s phenomenon. Cases have occurred with positive serologic testing (including positive anti-nuclear and/or anti-double-stranded DNA antibody testing). If BETASERON-treated patients develop new signs and symptoms characteristic of this syndrome, BETASERON therapy should be stopped.
Close5.12 Monitoring for Laboratory Abnormalities
In addition to those laboratory tests normally required for monitoring patients with multiple sclerosis, complete blood and differential white blood cell counts, platelet counts and blood chemistries, including liver function tests, are recommended at regular intervals (one, three, and six months) following introduction of BETASERON therapy, and then periodically thereafter in the absence of clinical symptoms.
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more details in other sections of labeling: • Hepatic Injury [see Warnings and Precautions (5.1)] • Anaphylaxis and Other Allergic ...
The following serious adverse reactions are discussed in more details in other sections of labeling:
- •
- Hepatic Injury [see Warnings and Precautions (5.1)]
- •
- Anaphylaxis and Other Allergic Reactions [see Warnings and Precautions (5.2)]
- •
- Depression and Suicide [see Warnings and Precautions (5.3)]
- •
- Congestive Heart Failure [see Warnings and Precautions (5.4)]
- •
- Injection Site Reactions Including Necrosis [see Warnings and Precautions (5.5)]
- •
- Leukopenia [see Warnings and Precautions (5.6)]
- •
- Thrombotic Microangiopathy [see Warnings and Precautions (5.7)]
- •
- Pulmonary Arterial Hypertension [see Warnings and Precautions (5.8)]
- •
- Flu-like Symptom Complex [see Warnings and Precautions (5.9)]
- •
- Seizures [see Warnings and Precautions (5.10)]
- •
- Drug Induced Lupus Erythematosus [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions and over varying lengths of time, adverse reaction rates observed in the clinical trials of BETASERON cannot be directly compared to rates in clinical trials of other drugs, and may not reflect the rates observed in practice.
Among 1407 patients with MS treated with BETASERON 0.25 mg every other day (including 1261 patients treated for greater than one year), the most commonly reported adverse reactions (at least 5% more frequent on BETASERON than on placebo) were injection site reaction, lymphopenia, flu-like symptoms, myalgia leukopenia, neutropenia, increased liver enzymes, headache, hypertonia, pain, rash, insomnia, abdominal pain, and asthenia. The most frequently reported adverse reactions resulting in clinical intervention (for example, discontinuation of BETASERON, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were depression, flu-like symptom complex, injection site reactions, leukopenia, increased liver enzymes, asthenia, hypertonia, and myasthenia.
Table 2 enumerates adverse reactions and laboratory abnormalities that occurred among patients treated with 0.25 mg of BETASERON every other day by subcutaneous injection in the pooled placebo-controlled trials (Study 1-4) at an incidence that was at least 2% more than that observed in the placebo-treated patients [see Clinical Studies (14)].
Table 2: Adverse Reactions and Laboratory Abnormalities in Patients with MS in Pooled Studies 1, 2, 3, and 4 Adverse Reaction Placebo
(N=965)BETASERON
(N=1407)- *
- "Injection site reaction" comprises all adverse reactions occurring at the injection site (except injection site necrosis), that is, the following terms: injection site reaction, injection site hemorrhage, injection site hypersensitivity, injection site inflammation, injection site mass, injection site pain, injection site edema and injection site atrophy.
- †
- "Flu-like symptom (complex)" denotes flu syndrome and/or a combination of at least two adverse reactions from fever, chills, myalgia, malaise, sweating.
Blood and lymphatic system disorders
Lymphocytes count decreased (<1500/mm3)
66%
86%
Absolute neutrophil count decreased (<1500/mm3)
5%
13%
White blood cell count decreased (<3000/mm3)
4%
13%
Lymphadenopathy
3%
6%
Nervous system disorders
Headache
43%
50%
Insomnia
16%
21%
Incoordination
15%
17%
Vascular disorders
Hypertension
4%
6%
Respiratory, thoracic and mediastinal disorders
Dyspnea
3%
6%
Gastrointestinal disorders
Abdominal pain
11%
16%
Hepatobiliary disorders
Alanine aminotransferase increased
(SGPT > 5 times baseline)4%
12%
Aspartate aminotransferase increased
(SGOT > 5 times baseline)1%
4%
Skin and subcutaneous tissue disorders
Rash
15%
21%
Skin disorder
8%
10%
Musculoskeletal and
connective tissue disorders
Hypertonia
33%
40%
Myalgia
14%
23%
Renal and urinary disorders
Urinary urgency
8%
11%
Reproductive system and breast disorders
Metrorrhagia
7%
9%
Impotence
6%
8%
General disorders and administration site conditions
Injection site reaction*
26%
78%
Asthenia
48%
53%
Flu-like symptoms (complex)†
37%
57%
Pain
35%
42%
Fever
19%
31%
Chills
9%
21%
Peripheral edema
10%
12%
Chest pain
6%
9%
Malaise
3%
6%
Injection site necrosis
0%
4%
In addition to the Adverse Reactions listed in Table 2, the following adverse reactions occurred more frequently on BETASERON than on placebo, but with a difference smaller than 2%: alopecia, anxiety, arthralgia, constipation, diarrhea, dizziness, dyspepsia, dysmenorrhea, leg cramps, menorrhagia, myasthenia, nausea, nervousness, palpitations, peripheral vascular disorder, prostatic disorder, tachycardia, urinary frequency, vasodilatation, and weight increase.
Laboratory Abnormalities
In the four clinical trials (Studies 1, 2, 3, and 4), leukopenia was reported in 18% and 6% of patients in BETASERON- and placebo-treated groups, respectively. No patients were withdrawn or dose reduced for neutropenia in Study 1. Three percent (3%) of patients in Studies 2 and 3 experienced leukopenia and were dose-reduced. Other abnormalities included increase of SGPT to greater than five times baseline value (12%), and increase of SGOT to greater than five times baseline value (4%). In Study 1, two patients were dose reduced for increased hepatic enzymes; one continued on treatment and one was ultimately withdrawn. In Studies 2 and 3, 1.5% of BETASERON patients were dose-reduced or interrupted treatment for increased hepatic enzymes. In Study 4, 1.7% of patients were withdrawn from treatment due to increased hepatic enzymes, two of them after a dose reduction. In Studies 1-4, nine (0.6%) patients were withdrawn from treatment with BETASERON for any laboratory abnormality, including four (0.3%) patients following dose reduction.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. Serum samples were monitored for the development of antibodies to BETASERON during Study 1. In patients receiving 0.25 mg every other day 56/124 (45%) were found to have serum neutralizing activity at one or more of the time points tested. In Study 4, neutralizing activity was measured every 6 months and at end of study. At individual visits after start of therapy, activity was observed in 17% up to 25% of the BETASERON-treated patients. Such neutralizing activity was measured at least once in 75 (30%) out of 251 BETASERON patients who provided samples during treatment phase; of these, 17 (23%) converted to negative status later in the study. Based on all the available evidence, the relationship between antibody formation and clinical safety or efficacy is not known.
These data reflect the percentage of patients whose test results were considered positive for antibodies to BETASERON using a biological neutralization assay that measures the ability of immune sera to inhibit the production of the interferon-inducible protein, MxA. Neutralization assays are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of neutralizing activity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to BETASERON with the incidence of antibodies to other products may be misleading.
Anaphylactic reactions have been reported with the use of BETASERON [see Warnings and Precautions (5.2)].
Close6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of BETASERON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Anemia, Thrombocytopenia, Hemolytic anemia
Endocrine disorders: Hypothyroidism, Hyperthyroidism, Thyroid dysfunction
Metabolism and nutrition disorders: Triglyceride increased, Anorexia, Weight decrease, Weight increase
Psychiatric disorders: Anxiety, Confusion, Emotional lability
Nervous system disorders: Convulsion, Dizziness, Psychotic symptoms
Cardiac disorders: Cardiomyopathy, Palpitations, Tachycardia
Vascular disorders: Vasodilatation
Respiratory, thoracic and mediastinal disorders: Bronchospasm, Pulmonary Arterial Hypertension
Gastrointestinal disorders: Diarrhea, Nausea, Pancreatitis, Vomiting
Hepatobiliary disorders: Hepatitis, Gamma GT increased
Skin and subcutaneous tissue disorders: Alopecia, Pruritus, Skin discoloration, Urticaria
Musculoskeletal and connective tissue disorders: Arthralgia
Reproductive system and breast disorder: Menorrhagia
General disorders and administration site conditions: Fatal capillary leak syndrome1
1The administration of cytokines to patients with a pre-existing monoclonal gammopathy has been associated with the development of this syndrome.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Although there have been no well-controlled studies in pregnant women, available data, which includes prospective observational studies, have not generally ...
8.1 Pregnancy
Risk Summary
Although there have been no well-controlled studies in pregnant women, available data, which includes prospective observational studies, have not generally indicated a drug-associated risk of major birth defects with interferon beta-1b during pregnancy. Administration of BETASERON to monkeys during gestation resulted in increased embryo-fetal death at or above exposures greater than 3 times the human therapeutic dose (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Human Data
The majority of the observational studies reporting on pregnancies exposed to interferon beta-1b did not identify an association between the use of interferon beta-1b during pregnancy and an increased risk of major birth defects
Animal Data
When BETASERON (doses ranging from 0.028 to 0.42 mg/kg/day) was administered to pregnant rhesus monkeys throughout the period of organogenesis (gestation days 20 to 70), a dose-related abortifacient effect was observed. The low-effect dose is approximately 3 times the recommended human dose of 0.25 mg on a body surface area (mg/m2) basis. A no-effect dose for embryo-fetal developmental toxicity in rhesus monkeys was not established.
8.2 Lactation
Risk Summary
There are no data on the presence of BETASERON in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BETASERON and any potential adverse effects on the breastfed child from BETASERON or from the underlying maternal condition.
Close8.5 Geriatric Use
Clinical studies of BETASERON did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
-
11 DESCRIPTIONInterferon beta-1b is a purified, sterile, lyophilized protein product produced by recombinant DNA techniques. Interferon beta-1b is manufactured by bacterial fermentation of a strain of ...
Interferon beta-1b is a purified, sterile, lyophilized protein product produced by recombinant DNA techniques. Interferon beta-1b is manufactured by bacterial fermentation of a strain of Escherichia coli that bears a genetically engineered plasmid containing the gene for human interferon betaser17. The native gene was obtained from human fibroblasts and altered in a way that substitutes serine for the cysteine residue found at position 17. Interferon beta-1b has 165 amino acids and an approximate molecular weight of 18,500 daltons. It does not include the carbohydrate side chains found in the natural material.
The specific activity of BETASERON is approximately 32 million international units (IU)/mg interferon beta-1b. Each vial contains 0.3 mg of interferon beta-1b. The unit measurement is derived by comparing the antiviral activity of the product to the World Health Organization (WHO) reference standard of recombinant human interferon beta. Mannitol, USP and Albumin (Human), USP (15 mg each/vial) are added as stabilizers.
Lyophilized BETASERON is a sterile, white to off-white powder, for subcutaneous injection after reconstitution with the diluent supplied (Sodium Chloride, 0.54% Solution). Albumin (Human) USP and Mannitol, USP (15 mg each/vial) are added as stabilizers.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of BETASERON (interferon beta-1b) in patients with multiple sclerosis is unknown. 12.2 Pharmacodynamics - Interferons (IFNs) are a family of ...
12.1 Mechanism of Action
The mechanism of action of BETASERON (interferon beta-1b) in patients with multiple sclerosis is unknown.
12.2 Pharmacodynamics
Interferons (IFNs) are a family of naturally occurring proteins, produced by eukaryotic cells in response to viral infection and other biologic agents. Three major types of interferons have been defined: type 1 (IFN-alpha, beta, epsilon, kappa and omega), type II (IFN–gamma) and type III (IFN-lambda). Interferon-beta is a member of the type I subset of interferons. The type I interferons have considerably overlapping but also distinct biologic activities. The bioactivities of all IFNs, including IFN-beta, are induced via their binding to specific receptors on the membranes of human cells. Differences in the bioactivities induced by the three major subtypes of IFNs likely reflect differences in the signal transduction pathways induced by signaling through their cognate receptors.
Interferon beta-1b receptor binding induces the expression of proteins that are responsible for the pleiotropic bioactivities of interferon beta-1b. A number of these proteins (including neopterin, β2-microglobulin, MxA protein, and IL-10) have been measured in blood fractions from BETASERON-treated patients and BETASERON-treated healthy volunteers. Immunomodulatory effects of interferon beta-1b include the enhancement of suppressor T cell activity, reduction of pro-inflammatory cytokine production, down-regulation of antigen presentation, and inhibition of lymphocyte trafficking into the central nervous system. It is not known if these effects play an important role in the observed clinical activity of BETASERON in multiple sclerosis (MS).
Close12.3 Pharmacokinetics
Because serum concentrations of interferon beta-1b are low or not detectable following subcutaneous administration of 0.25 mg or less of BETASERON, pharmacokinetic information in patients with MS receiving the recommended dose of BETASERON is not available.
Following single and multiple daily subcutaneous administrations of 0.5 mg BETASERON to healthy volunteers (N=12), serum interferon beta-1b concentrations were generally below 100 IU/mL. Peak serum interferon beta-1b concentrations occurred between one to eight hours, with a mean peak serum interferon concentration of 40 IU/mL. Bioavailability, based on a total dose of 0.5 mg BETASERON given as two subcutaneous injections at different sites, was approximately 50%.
After intravenous administration of BETASERON (0.006 mg to 2 mg), similar pharmacokinetic profiles were obtained from healthy volunteers (N=12) and from patients with diseases other than MS (N=142). In patients receiving single intravenous doses up to 2 mg, increases in serum concentrations were dose proportional. Mean serum clearance values ranged from 9.4 mL/min•kg-1 to 28.9 mL/min•kg-1 and were independent of dose. Mean terminal elimination half-life values ranged from 8 minutes to 4.3 hours and mean steady-state volume of distribution values ranged from 0.25 L/kg to 2.88 L/kg. Three-times-a-week intravenous dosing for two weeks resulted in no accumulation of interferon beta-1b in sera of patients. Pharmacokinetic parameters after single and multiple intravenous doses of BETASERON were comparable.
Following every other day subcutaneous administration of 0.25 mg BETASERON in healthy volunteers, biologic response marker levels (neopterin, β2- microglobulin, MxA protein, and the immunosuppressive cytokine, IL-10) increased significantly above baseline six-twelve hours after the first BETASERON dose. Biologic response marker levels peaked between 40 and 124 hours and remained elevated above baseline throughout the seven-day (168-hour) study. The relationship between serum interferon beta-1b levels or induced biologic response marker levels and the clinical effects of interferon beta-1b in multiple sclerosis is unknown.
Drug Interaction Studies
No formal drug interaction studies have been conducted with BETASERON.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - BETASERON has not been tested for its carcinogenic potential in animals. Mutagenesis - BETASERON was not genotoxic in ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
BETASERON has not been tested for its carcinogenic potential in animals.
Mutagenesis
BETASERON was not genotoxic in the in vitro Ames bacterial test or the in vitro chromosomal aberration assay in human peripheral blood lymphocytes. BETASERON treatment of mouse BALBc-3T3 cells did not result in increased transformation frequency in an in vitro model of tumor transformation.
Impairment of Fertility
Administration of BETASERON (doses of up to 0.33 mg/kg/day) to normally cycling female rhesus monkeys had no apparent adverse effects on either menstrual cycle duration or associated hormonal profiles (progesterone and estradiol) when administered over three consecutive menstrual cycles. The highest dose tested is approximately 30 times the recommended human dose of 0.25 mg on a body surface area (mg/m2) basis. The potential for other effects on fertility or reproductive performance was not evaluated.
-
14 CLINICAL STUDIESThe clinical effects of BETASERON were studied in four randomized, multicenter, double-blind, placebo-controlled studies in patients with multiple sclerosis (Studies 1, 2, 3, and 4). Patients ...
The clinical effects of BETASERON were studied in four randomized, multicenter, double-blind, placebo-controlled studies in patients with multiple sclerosis (Studies 1, 2, 3, and 4).
Patients with Relapsing-Remitting Multiple Sclerosis
The effectiveness of BETASERON in relapsing-remitting MS (RRMS) was evaluated in a double-blind, multiclinic, randomized, parallel, placebo controlled clinical study of two years duration (Study 1). The study enrolled MS patients, aged 18 to 50, who were ambulatory [Kurtzke Expanded Disability Status Scale (EDSS) of ≤ 5.5 – score 5.5 is ambulatory for 100 meters, disability precludes full daily activities], exhibited a relapsing-remitting clinical course, met Poser’s criteria for clinically definite and/or laboratory supported definite MS and had experienced at least two exacerbations over two years preceding the trial without exacerbation in the preceding month. The EDSS score is a method of quantifying disability in patients with MS and ranges from 0 (normal neurologic exam) to 10 (death due to MS). Patients who had received prior immunosuppressant therapy were excluded.
An exacerbation was defined as the appearance of a new clinical sign/symptom or the clinical worsening of a previous sign/symptom (one that had been stable for at least 30 days) that persisted for a minimum of 24 hours.
Patients selected for study were randomized to treatment with either placebo (N=123), 0.05 mg of BETASERON (N=125), or 0.25 mg of BETASERON (N=124) self-administered subcutaneously every other day. Outcome based on the 372 randomized patients was evaluated after two years.
Patients who required more than three 28-day courses of corticosteroids were removed from the study. Minor analgesics (acetaminophen, codeine), antidepressants, and oral baclofen were allowed ad libitum, but chronic nonsteroidal anti-inflammatory drug (NSAID) use was not allowed.
The primary protocol-defined outcome measures were 1) frequency of exacerbations per patient and 2) proportion of exacerbation free patients. A number of secondary clinical and magnetic resonance imaging (MRI) measures were also employed. All patients underwent annual T2 MRI imaging and a subset of 52 patients at one site had MRIs performed every six weeks for assessment of new or expanding lesions.
The study results are shown in Table 3.
Table 3: Two-Year RRMS Study Results of Primary and Secondary Clinical Outcomes (Study 1) Efficacy Parameters Treatment Groups Statistical Comparisons
p-valuePrimary End Points Placebo
(N=123)BETASERON 0.05 mg (N=125) BETASERON 0.25 mg (N=124) Placebo
vs
0.05 mg0.05 mg
vs
0.25 mgPlacebo
vs
0.25 mg- *
- 14 exacerbation free patients (0 from placebo, six from 0.05 mg, and eight from 0.25 mg) dropped out of the study before completing six months of therapy. These patients are excluded from this analysis.
- †
- Sequelae and Functional Neurologic Status, both required by protocol, were not analyzed individually but are included as a function of the EDSS.
- ‡
- EDSS scores range from 1-10, with higher scores reflecting greater disability.
- §
- Scripps neurologic rating scores range from 0-100, with smaller scores reflecting greater disability.
- ¶
- ND = Not done.
Annual exacerbation rate
1.31
1.14
0.9
0.005
0.113
0.0001
Proportion of exacerbation-free patients*
16%
18%
25%
0.609
0.288
0.094
Exacerbation frequency per patient
0*
1
2
3
4
> 5
20%
32%
20%
15%
15%
21%
22%
31%
28%
15%
7%
16%
29%
39%
17%
14%
9%
8%
0.151
0.077
0.001
Secondary Endpoints†
Median number of months to first on-study exacerbation
5
6
9
0.299
0.097
0.01
Rate of moderate or severe exacerbations per year
0.47
0.29
0.23
0.02
0.257
0.001
Mean number of moderate or severe exacerbation days per patient
44
33
20
0.229
0.064
0.001
Mean change in EDSS score‡ at endpoint
0.21
0.21
-0.07
0.995
0.108
0.144
Mean change in Scripps score§ at endpoint
-0.53
-0.5
0.66
0.641
0.051
0.126
Median duration in days per exacerbation
36
33
36
ND¶
ND¶
ND¶
% change in mean MRI lesion area at endpoint
21.4%
9.8%
-0.9%
0.015
0.019
0.0001
Of the 372 RRMS patients randomized, 72 (19%) failed to complete two full years on their assigned treatments.
Over the two-year period in Study 1, there were 25 MS-related hospitalizations in the 0.25 mg BETASERON-treated group compared to 48 hospitalizations in the placebo group. In comparison, non-MS hospitalizations were evenly distributed among the groups, with 16 in the 0.25 mg BETASERON group and 15 in the placebo group. The average number of days of MS-related steroid use was 41 days in the 0.25 mg BETASERON group and 55 days in the placebo group (p=0.004).
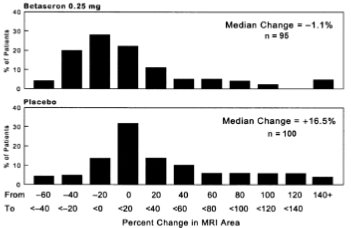
MRI data were also analyzed for patients in this study. A frequency distribution of the observed percent changes in MRI area at the end of two years was obtained by grouping the percentages in successive intervals of equal width. Figure 1 displays a histogram of the proportions of patients, which fell into each of these intervals. The median percent change in MRI area for the 0.25 mg group was -1.1%, which was significantly smaller than the 16.5% observed for the placebo group (p=0.0001).
Figure 1: Distribution of Change in MRI Area in Patients with RRMS in Study 1
In an evaluation of frequent MRI scans (every six weeks) on 52 patients at one site in Study 1, the percent of scans with new or expanding lesions was 29% in the placebo group and 6% in the 0.25 mg treatment group (p=0.006).
Patients with Secondary Progressive Multiple Sclerosis
Studies 2 and 3 were multicenter, randomized, double-blind, placebo controlled trials conducted to assess the effect of BETASERON in patients with secondary progressive MS (SPMS). Study 2 was conducted in Europe and Study 3 was conducted in North America. Both studies enrolled patients with clinically definite or laboratory-supported MS in the secondary progressive phase, and who had evidence of disability progression (both Study 2 and 3) or two relapses (Study 2 only) within the previous two years. Baseline Kurtzke expanded disability status scale (EDSS) scores ranged from 3.0 to 6.5. Patients in Study 2 were randomized to receive BETASERON 0.25 mg (N=360) or placebo (N=358). Patients in Study 3 were randomized to BETASERON 0.25 mg (N=317), BETASERON 0.16 mg/m2 of body surface area (N=314, mean assigned dose 0.3 mg), or placebo (N=308). Test agents were administered subcutaneously, every other day for three years.
The primary outcome measure was progression of disability, defined as a 1.0 point increase in the EDSS score, or a 0.5 point increase for patients with baseline EDSS ≥ 6.0. In Study 2, time to progression in EDSS was longer in the BETASERON treatment group (p=0.005), with estimated annualized rates of progression of 16% and 19% in the BETASERON and placebo groups, respectively. In Study 3, the rates of progression did not differ significantly between treatment groups, with estimated annualized rates of progression of 12%, 14%, and 12% in the BETASERON fixed dose, surface area-adjusted dose, and placebo groups, respectively.
Multiple analyses, including covariate and subset analyses based on sex, age, disease duration, clinical disease activity prior to study enrollment, MRI measures at baseline and early changes in MRI following treatment were evaluated in order to interpret the discordant study results. No demographic or disease-related factors enabled identification of a patient subset where BETASERON treatment was predictably associated with delayed progression of disability.
In Studies 2 and 3, like Study 1, a statistically significant decrease in the incidence of relapses associated with BETASERON treatment was demonstrated. In Study 2, the mean annual relapse rates were 0.42 and 0.63 in the BETASERON and placebo groups, respectively (p<0.001). In Study 3, the mean annual relapse rates were 0.16, 0.20, and 0.28, for the fixed dose, surface area-adjusted dose, and placebo groups, respectively (p<0.02).
MRI endpoints in both Study 2 and Study 3 showed smaller increases in T2 MRI lesion area and decreased number of active MRI lesions in patients in the BETASERON groups compared to the placebo group.
Patients with an Isolated Demyelinating Event and Typical MS Lesions on Brain MRI
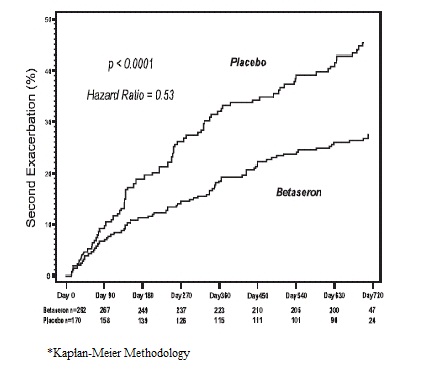
In Study 4, 468 patients who had recently (within 60 days) experienced an isolated demyelinating event, and who had lesions typical of multiple sclerosis on brain MRI were randomized to receive either 0.25 mg BETASERON (N = 292) or placebo (N=176) subcutaneously every other day (ratio 5:3). The primary outcome measure was time to development of a second exacerbation with involvement of at least two distinct anatomical regions. Secondary outcomes were brain MRI measures, including the cumulative number of newly active lesions, and the absolute change in T2 lesion volume. Patients were followed for up to two years or until they fulfilled the primary endpoint.
Eight percent of subjects on BETASERON and 6% of subjects on placebo withdrew from the study for a reason other than the development of a second exacerbation. Time to development of a second exacerbation was significantly delayed in patients treated with BETASERON compared to patients treated with placebo (p<0.0001). The Kaplan-Meier estimates of the percentage of patients developing an exacerbation within 24 months were 45% in the placebo group and 28% of the BETASERON group (Figure 2). The risk for developing a second exacerbation in the BETASERON group was 53% of the risk in the placebo group (Hazard ratio= 0.53; 95% confidence interval 0.39 to 0.73).
Figure 2: Onset of Second Exacerbation by Time in Patients with Isolated Demyelinating Event with Typical MS Lesions on Brain MRI in Study 4*
In Study 4, patients treated with BETASERON demonstrated a lower number of newly active lesions during the course of the study. A significant difference between BETASERON and placebo was not seen in the absolute change in T2 lesion volume during the course of the study.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - BETASERON is supplied as a lyophilized powder in a clear glass, single-dose vial (3 mL capacity). Each carton contains 5 single-dose cartons (NDC 50419-524-05) or 14 ...
16.1 How Supplied
BETASERON is supplied as a lyophilized powder in a clear glass, single-dose vial (3 mL capacity). Each carton contains 5 single-dose cartons (NDC 50419-524-05) or 14 single-dose cartons (NDC 50419-524-35).
Each single-dose carton contains:
A single-dose vial containing 0.3 mg BETASERON (interferon beta-1b)
A pre-filled single-dose syringe containing 1.2 mL diluent (Sodium Chloride, 0.54% solution)
A vial adapter with a 30-gauge needle attached
2 alcohol prep pads
The optional BETACONNECT autoinjector is not supplied with BETASERON, but is available for patients with a prescription for BETASERON by calling the BETAPLUS patient support program toll-free number at 1-800-788-1467.
Close16.2 Stability and Storage
BETASERON and the diluent are for single-dose only. Discard unused portions. The reconstituted product contains no preservative. Store BETASERON vials between 36°F to 86°F (2°C to 30°C). After reconstitution, if not used immediately, refrigerate the reconstituted solution and use within three hours. Do not freeze.
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients to carefully read the supplied BETASERON Medication Guide and caution patients not to change the ...
See FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients to carefully read the supplied BETASERON Medication Guide and caution patients not to change the BETASERON dose or schedule of administration without medical consultation.
Instruction on Self-Injection Technique and Procedures
Provide appropriate instruction for reconstitution of BETASERON and methods of self-injection, including careful review of the BETASERON Medication Guide. Instruct patients in the use of aseptic technique when administering BETASERON.
Tell patients not to re-use needles or syringes and instruct patients on safe disposal procedures. Advise patients of the importance of rotating areas of injection with each dose, to minimize the likelihood of severe injection site reactions, including necrosis or localized infection [see Medication Guide].
Hepatic Injury
Advise patients that severe hepatic injury, including hepatic failure, has been reported during the use of BETASERON.
Inform patients of symptoms of hepatic dysfunction, and instruct patients to report them immediately to their healthcare provider [see Warnings and Precautions (5.1)].
Anaphylaxis and Other Allergic Reactions
Advise patients of the symptoms of allergic reactions and anaphylaxis, and instruct patients to seek immediate medical attention if these symptoms occur [see Warnings and Precautions (5.2)].
Depression and Suicide
Advise patients that depression and suicidal ideation have been reported during the use of BETASERON. Inform patients of the symptoms of depression or suicidal ideation, and instruct patients to report them immediately to their healthcare provider [see Warnings and Precautions (5.3)].
Congestive Heart Failure
Advise patients that worsening of pre-existing congestive heart failure have been reported in patients using BETASERON.
Advise patients of symptoms of worsening cardiac condition, and instruct patients to report them immediately to their healthcare provider [see Warnings and Precautions (5.4)].
Injection Site Reactions Including Necrosis
Advise patients that injection site reactions occur in most patients treated with BETASERON, and that injection site necrosis may occur at one or multiple sites. Instruct patients to promptly report any break in the skin, which may be associated with blue-black discoloration, swelling, or drainage of fluid from the injection site, prior to continuing their BETASERON therapy [see Warnings and Precautions (5.5)].
Pulmonary Arterial Hypertension
Inform patients that PAH has occurred in patients treated with interferon beta products, including BETASERON. Instruct patients to promptly report any new symptoms such as new or increasing fatigue or shortness of breath to their healthcare provider [see Warnings and Precautions (5.8)].
Flu-like Symptom Complex
Inform patients that flu-like symptoms are common following initiation of therapy with BETASERON, and that concurrent use of analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms associated with BETASERON use [see Warnings and Precautions (5.9) and Dosage and Administration (2.4)].
Seizures
Instruct patients to report seizures immediately to their healthcare provider [see Warnings and Precautions (5.10)].
Pregnancy
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant [see Use in Specific Populations (8.1)].
Close -
Medication Guide Medication Guide - BETASERON - (bay-ta-seer-on) interferon beta-1b - (in-ter-feer-on beta-one-be) What is the most important information I should know about ...
Medication Guide
BETASERON
(bay-ta-seer-on)interferon beta-1b
(in-ter-feer-on beta-one-be)What is the most important information I should know about BETASERON?
BETASERON can cause serious side effects, including:
- •
- liver problems including liver failure. Symptoms of liver problems may include:
- •
- yellowing of your eyes
- •
- itchy skin
- •
- nausea or vomiting
- •
- feeling very tired
- •
- flu-like symptoms
- •
- bruising easily or bleeding problems
Your healthcare provider will do blood tests to check for these problems while you take BETASERON.
- •
- serious allergic reactions. Serious allergic reactions can happen quickly and may happen after your first dose of BETASERON or after you have taken BETASERON many times. Symptoms may include difficulty breathing or swallowing, swelling of the mouth or tongue, rash, itching, or skin bumps.
- •
- depression or suicidal thoughts. Call your healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
- •
- thoughts about suicide or dying
- •
- new or worse depression
- •
- new or worse anxiety
- •
- trouble sleeping (insomnia)
- •
- acting aggressive, being angry, or violent
- •
- acting on dangerous impulses
- •
- hallucinations
- •
- other unusual changes in behavior or mood
What is BETASERON?
BETASERON is a prescription medicine used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. BETASERON is similar to certain interferon proteins that are produced in the body.
It is not known if BETASERON is safe and effective in children.
Who should not take BETASERON?
Do not take BETASERON if you are allergic to interferon beta-1b, to another interferon beta, to human albumin, or mannitol. See the end of this leaflet for a complete list of ingredients in BETASERON.
What should I tell my healthcare provider before taking BETASERON?
Before you take BETASERON, tell your healthcare provider if you:
- •
- have or have had depression (sinking feeling or sadness), anxiety (feeling uneasy, nervous, or fearful for no reason) or trouble sleeping
- •
- have or have had liver problems
- •
- have or have had blood problems such as bleeding or bruising easily, low red blood cells (anemia) or low white blood cells
- •
- have or have had seizures
- •
- have or have had heart problems
- •
- are pregnant or plan to become pregnant.
- •
- are breastfeeding or plan to breastfeed. It is not known if BETASERON passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take BETASERON?
- •
- See the Instructions for Use at the end of this Medication Guide for complete information on how to use BETASERON.
- •
- BETASERON is given by injection under your skin (subcutaneous injection) every other day.
- •
- Take BETASERON exactly as your healthcare provider tells you to take it.
- •
- If your healthcare provider feels that you or someone else may give you the injections then you or the other person should be trained by your healthcare provider in how to give an injection.
- •
- Do not try to give yourself or have another person give you injections until you or both of you understand and are comfortable with how to prepare your dose and give the injection.
- •
- You may be started on a lower dose when you first start taking BETASERON. Your healthcare provider will tell you what dose of BETASERON to use.
- •
- Your healthcare provider may change your dose of BETASERON. You should not change your dose without talking to your healthcare provider.
- •
- If you miss a dose, you should take your next dose as soon as you remember or are able to take it. Your next injection should be taken about 48 hours (2 days) after that dose. Do not take BETASERON on 2 consecutive days. If you accidentally take more than your prescribed dose, or take it on 2 consecutive days, call your healthcare provider right away.
- •
- Always use a new, unopened vial of BETASERON and pre-filled diluent syringe for each injection. Throw away any unused medicine. Do not re-use any vials, syringes, or needles.
- •
- It is important for you to change your injection site each time you inject BETASERON. This will lessen the chance of you having a serious skin reaction at the site where you inject BETASERON. Avoid injecting BETASERON into an area of skin that is sore, reddened, infected or has other problems.
What are the possible side effects of BETASERON?
BETASERON may cause serious side effects. Call your healthcare provider right away if you have any of the serious side effects of BETASERON including:
- •
- See “What is the most important information I should know about BETASERON?”
- •
- heart problems. BETASERON may worsen heart problems including congestive heart failure. Symptoms of heart problems may include:
- •
- swollen ankles
- •
- shortness of breath
- •
- not being able to lay flat in bed
- •
- tightness in chest
- •
- decreased ability to exercise
- •
- fast heartbeat
- •
- increased need to urinate at night
- •
- injection site problems. Serious skin reactions can happen in some people including areas of severe damage to skin and the tissue below the skin (necrosis). These reactions can happen anywhere you inject BETASERON. Symptoms of injection site problems may include swelling, redness, or pain at the injection site, fluid drainage from the injection site, and breaks in your skin or blue-black skin discoloration. Call your healthcare provider right away if an injection site becomes swollen and painful or the area looks infected. You may have a skin infection or an area of severe skin damage (necrosis) requiring treatment by a healthcare provider.
- •
- Pulmonary arterial hypertension. Pulmonary arterial hypertension can occur with interferon beta products, including BETASERON. Symptoms may include new or increasing fatigue or shortness of breath. Contact your healthcare provider right away if you develop these symptoms.
- •
- flu-like symptoms. BETASERON can cause flu-like symptoms including:
- o
- fever
- o
- tiredness
- o
- sweating
- o
- chills
- o
- muscle aches when you first start to use it
These symptoms may decrease over time. Taking medicines for fever and pain relief on the days you are using BETASERON may help decrease these symptoms.
- •
- seizures. Some people have had seizures while taking BETASERON, including people who have never had seizures before. It is not known if the seizures were related to their MS, to BETASERON, or to a combination of both. If you have a seizure after taking BETASERON call your healthcare provider right away.
The most common side effects of BETASERON include:
- o
- low white blood cell count
- o
- increases in your liver enzymes
- o
- problems sleeping
- o
- headache
- o
- increases in your muscle tension
- o
- weakness
- o
- pain
- o
- rash
- o
- stomach pain
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
- These are not all the possible side effects of BETASERON. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BETASERON?
- •
- Before mixing, store BETASERON between 36°F to 86°F (2°C to 30°C).
- •
- After mixing, you can refrigerate BETASERON for up to 3 hours before using. Your BETASERON must be used within 3 hours of mixing even if refrigerated.
- •
- Do not freeze.
Keep BETASERON and all medicines out of the reach of children.
General information about the safe and effective use of BETASERON.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BETASERON for a condition for which it was not prescribed. Do not give BETASERON to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about BETASERON. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about BETASERON that is written for health professionals.
For more information, go to www.BETASERON.com or call BETAPLUS, the BETASERON patient support program, at 1-800-788-1467.
What are the ingredients in BETASERON?
Active ingredient: interferon beta-1b
Inactive ingredients: albumin (human), mannitol
Diluent contains sodium chloride solution.
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 7/2023
Close -
Instructions for Use BETASERON - (bay-ta-seer-on) interferon beta-1b - (in-ter-feer-on beta-one-be) Read the Instructions for Use that come with your BETASERON before you start using it and each time you get a ...
BETASERON
(bay-ta-seer-on)interferon beta-1b
(in-ter-feer-on beta-one-be)Read the Instructions for Use that come with your BETASERON before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment. Before you use BETASERON for the first time, make sure your healthcare provider shows you the right way to use it.
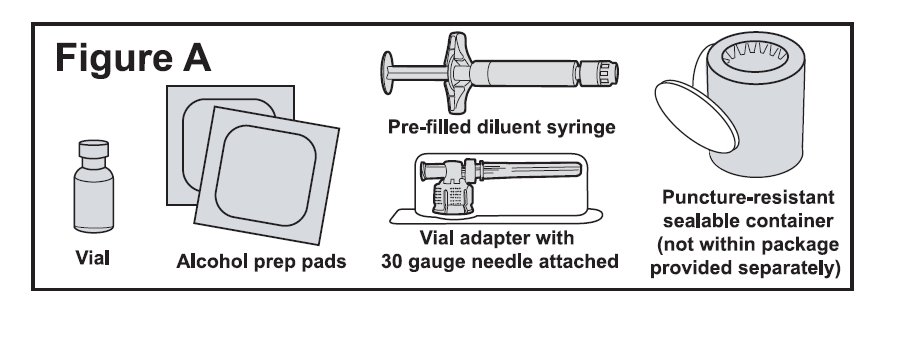
Supplies needed for your BETASERON Injection (See Figure A).
- •
- 1 single-dose carton containing:
- o
- A vial of BETASERON
- o
- A pre-filled diluent syringe
- o
- A vial adapter with a 30-gauge needle attached (in the blister pack)
- o
- 2 alcohol prep pads
Step 1: Preparing for Your BETASERON Injection
- •
- Place the supplies you will need on a clean, flat surface in a well-lit area.
- •
- Check the expiration date on the single-dose carton to make sure that it has not expired. Do not use it if the medication has expired.
- •
- Wash your hands thoroughly with soap and water.
- •
- Open the single-dose carton and take out all the contents. Make sure the blister pack containing the vial adapter is sealed. Check to make sure the plastic cap on the pre-filled diluent syringe is firmly attached.
- •
- Remove the tray from the single-dose carton and place it on a flat surface.
- •
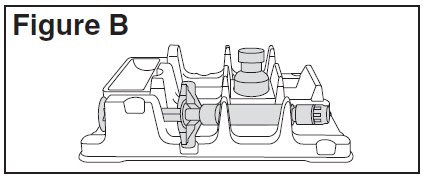
- Place the BETASERON vial in the well (vial holder) and place the pre-filled diluent syringe in the U-shaped trough (See Figure B).
Step 2: Mixing BETASERON
- •
- Remove the BETASERON vial from the well (vial holder) and take the cap off the vial.
- •
- Place the vial back into the well (vial holder).
- •
- Use an alcohol prep pad to clean the top of the vial. Move the alcohol prep pad in 1 direction. Leave the alcohol prep pad on top of the vial.
- •
- Peel the label off the blister pack with the vial adapter in it. The vial adapter is sterile. Do not remove or touch the vial adapter.
- •
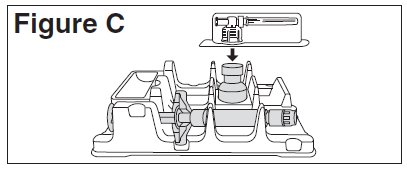
- Remove the alcohol prep pad from the top of the BETASERON vial. Pick up the vial adapter in the blister pack. Turn over the blister pack keeping the vial adapter inside. Put the adapter on top of the BETASERON vial. Push down on the adapter until it pierces the rubber top of the BETASERON vial and snaps in place (See Figure C). Remove the blister packaging from the vial adapter.
- •
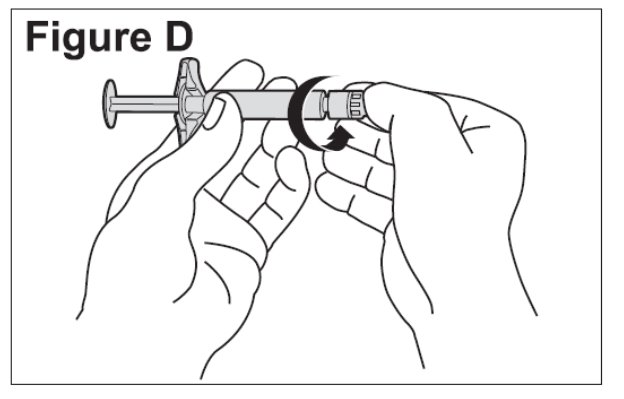
- Twist the plastic cap from the pre-filled diluent syringe. Throw away the plastic cap (See Figure D).
- •
- Keep the vial adapter attached to the vial and remove the vial from the well (vial holder). Be careful not to pull the vial adapter off the top of the vial.
- •
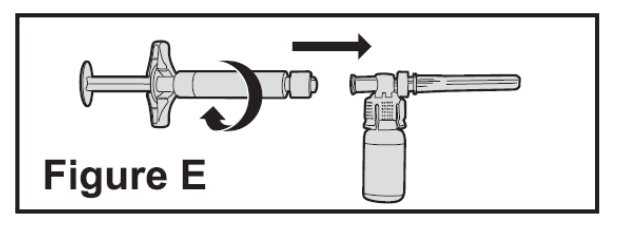
- Connect the pre-filled diluent syringe to the vial adapter by turning clockwise until resistance is felt and the attachment is secure. This forms the syringe assembly (See Figure E).
- •
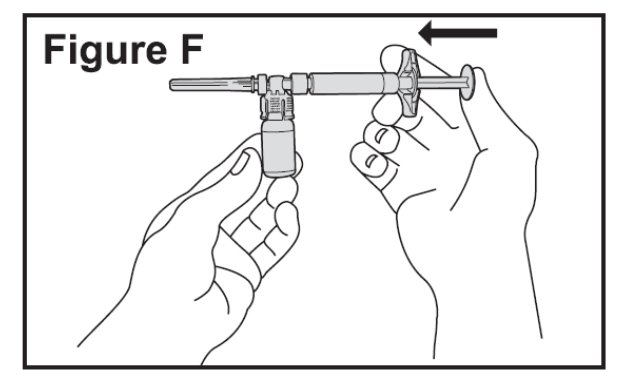
- Slowly push the plunger of the pre-filled diluent syringe all the way in. This will transfer all of the liquid from the syringe into the BETASERON vial (See Figure F). The plunger may return to its original position after you release it.
- •
- Gently swirl the vial to completely dissolve the white powder of BETASERON. Do not shake. Shaking and even gentle mixing can cause foaming of the medicine. If there is foam, let the vial sit until the foam settles before using it.
- •
- After the powder dissolves, look closely at the solution in the vial. Do not use the solution if it is cloudy or contains particles. It should be clear and colorless.
- •
- Do not use cracked or damaged BETASERON vials. If your vial is cracked or damaged, get a new single-dose carton containing a BETASERON vial, pre-filled diluent syringe, vial adapter and 2 alcohol prep pads. Repeat the steps to prepare your BETASERON dose.
- •
- Contact BETAPLUS, the BETASERON patient support program, at 1-800-788-1467 to obtain a replacement product.
Step 3: Preparing the Injection
You have completed the steps to prepare your BETASERON and are ready for the injection. The injection should be given immediately after mixing and allowing any foam in the solution to settle. If you must wait to give yourself the injection, you may refrigerate the solution and use within 3 hours of mixing your BETASERON. Do not freeze.
- •
- Push the plunger in and hold it there; then turn the syringe assembly so that the syringe is horizontal and the vial is on top.
- •
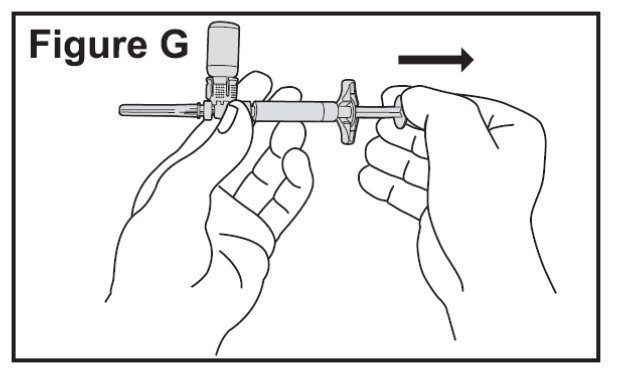
- Slowly pull the plunger back to withdraw all the liquid from the BETASERON vial into the syringe (See Figure G).
- •
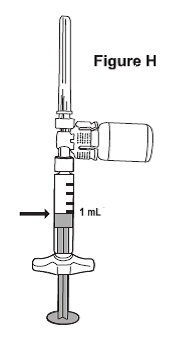
- NOTE: The syringe barrel is marked with numbers from 0.25 mL to 1 mL (See Figure H). If the solution in the vial cannot be drawn up to the 1 mL mark, discard the vial and syringe and start over with a new single-dose carton containing a BETASERON vial, pre-filled diluent syringe, vial adapter and alcohol prep pads.
- •
- Turn the syringe assembly so that the needle end is pointing up. Remove any air bubbles by tapping the outside of the syringe with your fingers. Slowly push the plunger to the 1 mL mark on the syringe or to the mark that matches the amount of BETASERON prescribed by your healthcare provider (See Figure H). If too much solution is pushed into the vial, repeat Step 3.
- •
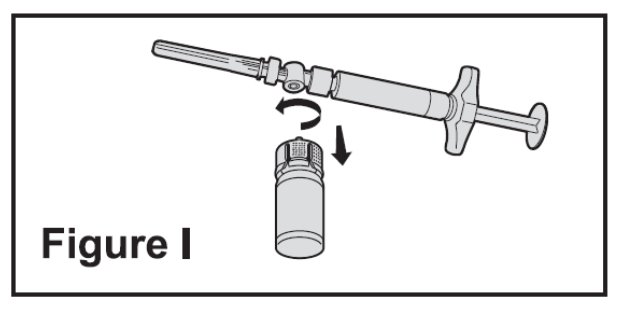
- Turn the syringe assembly so that the vial is at the bottom. Remove the vial adapter and the vial from the syringe by twisting the vial adapter. This will remove the vial adapter and the vial from the syringe, but will leave the needle on the syringe (See Figure I).
Step 4: Choosing an Injection Site
- •
- BETASERON (interferon beta-1b) is injected under the skin and into the fat layer between the skin and the muscles (subcutaneous tissue). The best areas for injection are where the skin is loose and soft and away from the joints, nerves, and bones. Do not use the area near your navel or waistline. If you are very thin, use only the thigh or outer surface of the arm for injection.
- •
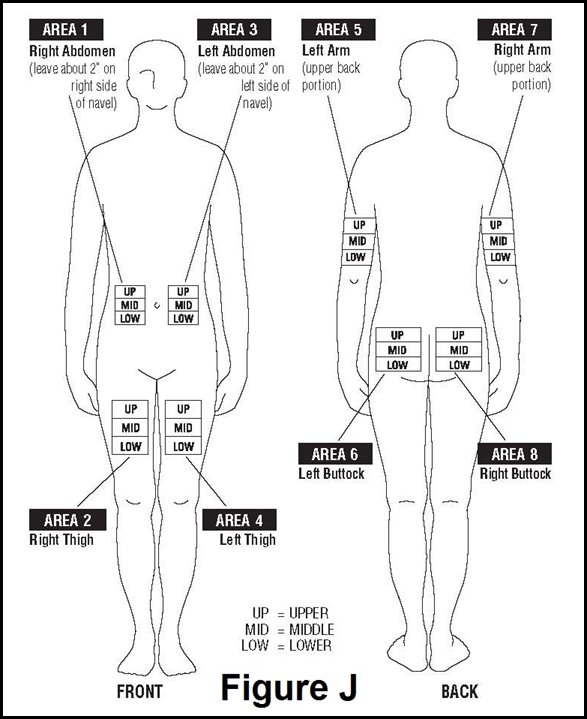
- Choose a different site each time you give yourself an injection. Figure J shows different areas for giving injections. Do not inject in the same area for 2 injections in a row. Keep a record of your injections to help make sure you change (rotate) your injection sites. You should decide where you will inject BETASERON before you prepare your medicine for injection. If there are any sites that are difficult for you to reach, you can ask someone who has been trained to give the injection to help you.
- •
- Do not inject BETASERON in a site where the skin is red, bruised, infected, or scabbed, has broken open, or has lumps, bumps, or pain. Tell your healthcare provider if you find skin conditions like the ones mentioned here or any other unusual-looking areas where you have been given injections.
Step 5: Injecting BETASERON
- •
- Using a circular motion, clean the injection site with an alcohol prep pad, starting at the injection site and moving outward. Let the skin area air dry.
- •
- Remove the cap from the needle. Hold the syringe like a pencil or dart in 1 hand.
- •
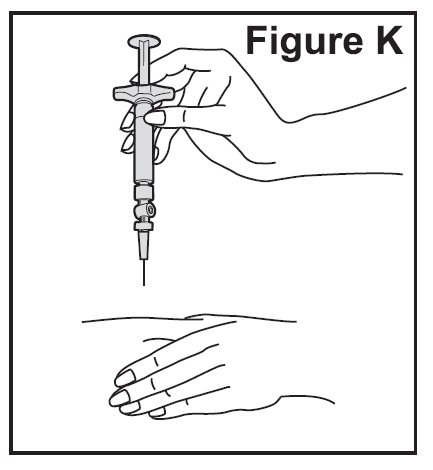
- Gently pinch the skin around the site with the thumb and forefinger of the other hand (See Figure K). Insert the needle straight up and down into your skin at a 90° angle with a quick, dart-like motion.
- •
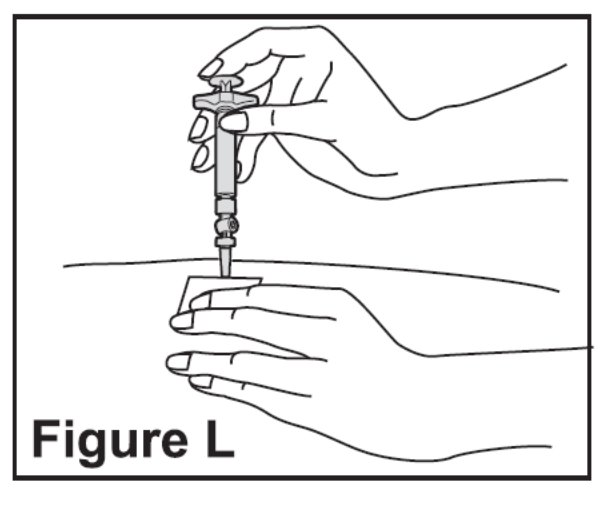
- Slowly push the plunger all the way in until the syringe is empty (See Figure L).
- •
- Remove the needle from the skin. Place a dry cotton ball or gauze pad over the injection site. Gently massage the injection site for a few moments with the dry cotton ball or gauze pad. Throw away the syringe in your puncture-proof disposal container.
- •
- Optional Use of BETACONNECT Autoinjector:
You may also give BETASERON by using the BETACONNECT autoinjector. You should get help with training on the use of the BETACONNECT autoinjector from a healthcare provider before using it for the first time. The BETACONNECT autoinjector should only be used with the syringes that come in the BETASERON packaging. See the Instructions for Use that come with the BETACONNECT autoinjector. For more information, call BETAPLUS, the BETASERON patient support program, at 1-800-788-1467.
Step 6: Disposing of used syringes, needles, and vials
- •
- To prevent needle-stick injury and spread of infection, do not try to re-cap the needle.
- •
- Place used needles, syringes, and vials in a closeable, puncture-resistant container. You may use a sharps container (such as a red biohazard container), hard plastic container (such as a detergent bottle), or metal container (such as an empty coffee can). Do not use glass or clear plastic containers. Ask your healthcare provider for instructions on the right way to throw away (dispose of) the container. There may be state and local laws about how you should throw away used needles and syringes.
- •
- Do not throw used needles, syringes, or vials in your household trash or recycle.
- •
- Throw away any unused medicine. Do not save any unused BETASERON for a future dose.
- •
- Keep the disposal container, needles, syringes, and vials of BETASERON out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration 11/2021
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981Manufactured in Germany
U.S. License No. 1778
© 1993 Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
Close -
PRINCIPAL DISPLAY PANELThe principal display panel is a representative example and may not reflect the most current label. NDC 50419-524-01 Rx only - BETASERON - (interferon beta-1b) For injection - 0.3 mg per vial - For ...
- The principal display panel is a representative example and may not reflect the most current label.
- NDC 50419-524-01 Rx only
BETASERON
(interferon beta-1b)For injection
0.3 mg per vial
For subcutaneous injection
No U.S. standard of potency
Single use carton
Contains:
- •
- 1 single-use vial for reconstitution
- •
- 1 pre-filled single-use sodium chloride 0.54% solution diluent syringe
- •
- 1 vial adapter with a 30 gauge needle attached
- •
- 2 alcohol prep pads
-
INGREDIENTS AND APPEARANCEProduct Information
BETASERON interferon beta-1b kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50419-524 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50419-524-35 14 in 1 BOX 08/11/2009 1 NDC:50419-524-01 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:50419-524-05 5 in 1 BOX 08/11/2009 2 NDC:50419-524-01 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:50419-524-09 1 in 1 BOX 08/11/2009 3 NDC:50419-524-01 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 4 NDC:50419-524-81 14 in 1 BOX 09/01/2016 4 NDC:50419-524-01 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 1 mL Part 2 1 SYRINGE 1.2 mL Part 1 of 2 BETASERON interferon beta-1b injection, powder, lyophilized, for solution Product Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INTERFERON BETA-1B (UNII: TTD90R31WZ) (INTERFERON BETA-1B - UNII:TTD90R31WZ) INTERFERON BETA-1B 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) 15 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 15 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103471 07/09/2009 Part 2 of 2 DILUENT sodium chloride solution, concentrate Product Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 5.4 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.2 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103471 07/09/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103471 07/09/2009 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH and Co. KG 340700520 MANUFACTURE(50419-524) , API MANUFACTURE(50419-524) , ANALYSIS(50419-524)
CloseEstablishment Name Address ID/FEI Business Operations Bayer AG 315097875 ANALYSIS(50419-524) , API MANUFACTURE(50419-524) , LABEL(50419-524) , MANUFACTURE(50419-524) , PACK(50419-524) , STERILIZE(50419-524)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
BETASERON- interferon beta-1b kit
Number of versions: 22
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Aug 21, 2024 | 23 (current) | download |
| Aug 14, 2023 | 22 | download |
| Aug 10, 2023 | 20 | download |
| Dec 8, 2022 | 19 | download |
| Nov 17, 2021 | 18 | download |
| Apr 5, 2021 | 17 | download |
| Oct 5, 2020 | 16 | download |
| Aug 20, 2019 | 15 | download |
| Jul 10, 2019 | 14 | download |
| Jul 8, 2019 | 13 | download |
| Sep 7, 2018 | 12 | download |
| Aug 9, 2017 | 11 | download |
| May 1, 2017 | 10 | download |
| Sep 16, 2016 | 9 | download |
| Jul 18, 2016 | 8 | download |
| May 16, 2016 | 7 | download |
| Apr 22, 2016 | 6 | download |
| Dec 28, 2015 | 5 | download |
| Dec 7, 2015 | 4 | download |
| Sep 28, 2015 | 3 | download |
| Feb 18, 2014 | 2 | download |
| Sep 18, 2013 | 1 | download |
RxNorm
BETASERON- interferon beta-1b kit
Get Label RSS Feed for this Drug
BETASERON- interferon beta-1b kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=66311f74-0472-4fa3-848a-06002ca0def5
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
BETASERON- interferon beta-1b kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50419-524-01 |
| 2 | 50419-524-05 |
| 3 | 50419-524-09 |
| 4 | 50419-524-35 |
| 5 | 50419-524-81 |