Label: SYNOVEX H- testosterone propionate and estradiol benzoate implant
- NDC Code(s): 54771-3901-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated July 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
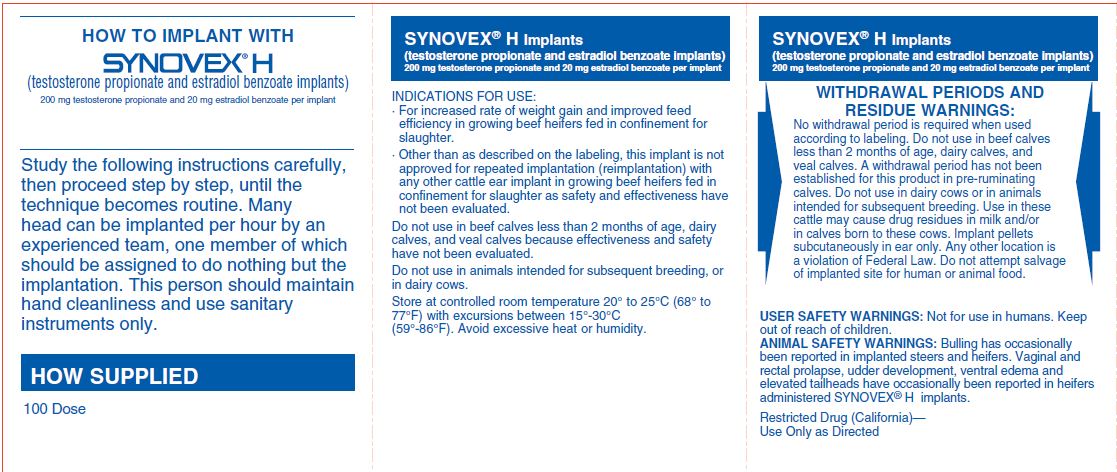
Synovex® H
(testosterone propionate and estradiol benzoate implants)
200 mg testosterone propionate and 20 mg estradiol benzoate per implant
SUBCUTANEOUS IMPLANTS FOR GROWING BEEF HEIFERS FED IN CONFINEMENT FOR SLAUGHTER
This product was manufactured by a non-sterilizing process.
TAKE TIME OBSERVE LABEL DIRECTIONS
Important: Read ALL sides of carton
For subcutaneous ear implantation only.
Approved by FDA under NADA # 011-427NET CONTENTS: 10 CARTRIDGES
Each Cartridge Contains 10 implants (100 implants total)Restricted Drug (California) — Use Only as Directed
-
WITHDRAWAL PERIODS AND RESIDUE WARNINGS
No withdrawal period is required when used according to labeling. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Implant pellets subcutaneously in ear only. Any other location is a violation of Federal Law.
Do not attempt salvage of implanted site for human or animal food. - USER SAFETY WARNINGS
- ANIMAL SAFETY WARNINGS
-
INDICATIONS FOR USE
· For increased rate of weight gain and improved feed efficiency in growing beef heifers fed in confinement for slaughter.
· Other than as described on the labeling, this implant is not approved for repeated implantation (reimplantation) with any other cattle ear implant in growing beef heifers fed in confinement for slaughter as safety and effectiveness have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been evaluated.
Do not use in animals intended for subsequent breeding, or in dairy cows. - Dosage
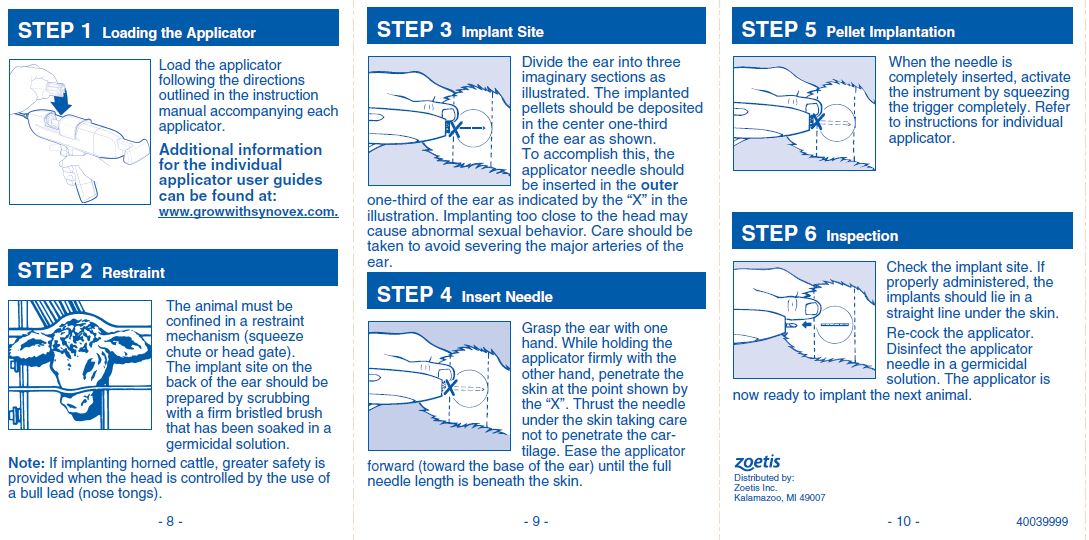
- DIRECTIONS
- Storage
- HOW SUPPLIED
- QUESTIONS/COMMENTS?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Carton of 10 cartridges
-
INGREDIENTS AND APPEARANCE
SYNOVEX H
testosterone propionate and estradiol benzoate implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-3901 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE PROPIONATE (UNII: WI93Z9138A) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE PROPIONATE 200 mg ESTRADIOL BENZOATE (UNII: 1S4CJB5ZGN) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL BENZOATE 20 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3901-1 1 in 1 BOX 1 10 in 1 POUCH 1 10 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA011427 11/07/2014 Labeler - Zoetis Inc. (828851555)