Label: PREDNICARBATE ointment
- NDC Code(s): 0168-0410-15, 0168-0410-60
- Packager: Fougera Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Prednicarbate ointment 0.1% contains the non-halogenated prednisolone derivative prednicarbate. The topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents.
Each gram of prednicarbate ointment 0.1% contains 1.0 mg of prednicarbate USP in a base consisting of white petrolatum, glyceryl monooleate, octyldodecanol NF, and propylene glycol.
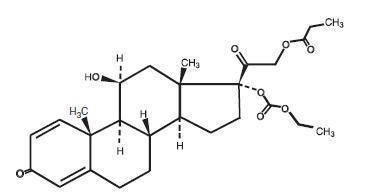
Prednicarbate has the empirical formula C27H36O8 and a molecular weight of 488.58.
The CAS Registry Number is 73771-04-7.
The chemical structure is:
-
CLINICAL PHARMACOLOGY:
Like other topical corticosteroids, prednicarbate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin while inflammation and/or other disease processes in the skin increase percutaneous absorption. Studies performed with prednicarbate ointment 0.1% indicate that it is in the medium range of potency as compared with other topical corticosteroids.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
PRECAUTIONS:
- General: Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment. Patients receiving a large dose of a higher potency topical steroid applied to a large surface area or under occlusion should be evaluated periodically for evidence of HPA-axis suppression. This may be done by using the ACTH stimulation, A.M. plasma cortisol, and urinary free cortisol tests. Prednicarbate ointment 0.1% did not produce significant HPA-axis suppression when used at a dose of 60 grams per day for a week in patients with extensive psoriasis or atopic dermatitis. However, if HPA-axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid. Recovery of HPA-axis function is generally prompt and complete upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products. Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface area to body mass ratios (See PRECAUTIONS - Pediatric Use .) If irritation develops, prednicarbate ointment 0.1% should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noting a clinical exacerbation, as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing. If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of prednicarbate ointment 0.1% should be discontinued until the infection has been adequately controlled.
Information for Patients: Patients using topical corticosteroids should receive the following information and instructions:
- 1.
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- 2.
- This medication should not be used for any disorder other than that for which it was prescribed.
- 3.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive, unless directed by the physician.
- 4.
- Patients should report to their physician any signs of local adverse reactions.
- 5.
- This medication should not be used on the face, underarms, or groin areas.
- 6.
- Contact between Prednicarbate Ointment 0.1% and latex containing products (eg. condoms, diaphragm etc.) should be avoided since paraffin in contact with latex can cause damage and reduce the effectiveness of any latex containing products. If latex products come into contact with Prednicarbate Ointment 0.1%, patients should be advised to discard the latex products. Patients should be advised that this medication is to be used externally only, not intravaginally.
Laboratory Tests: The following tests may be helpful in evaluating patients for HPA axis suppression: ACTH stimulation test, A.M. plasma cortisol test, Urinary free cortisol test.
Carcinogenesis, Mutagenesis, Impairment of Fertility: In a study of the effect of prednicarbate on fertility, pregnancy and postnatal development in rats, no effect was noted on the fertility or pregnancy of the parent animals or postnatal development of the offspring after administration of up to 0.80 mg/kg of prednicarbate subcutaneously. Prednicarbate has been evaluated in the Salmonella reversion test (Ames test) over a wide range of concentrations in the presence and absence of an S-9 liver microsomal fraction and did not demonstrate mutagenic activity. Similarly, prednicarbate did not produce any significant changes in the numbers of micronuclei seen in erythrocytes when mice were given doses ranging from 1 to 160 mg/kg of the drug.
Pregnancy: Teratogenic effects: Pregnancy Category C: Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Prednicarbate has been shown to be teratogenic and embryotoxic in Wistar rats and Himalayan rabbits when given subcutaneously during gestation at doses 1900 times and 45 times, respectively, the recommended topical human dose, assuming a percutaneous absorption of approximately 3%. In the rats, slightly retarded fetal development and an incidence of thickened and wavy ribs which were higher than the spontaneous rates were noted.
In rabbits, there was noted increased liver weights and slight increase in the fetal intrauterine death rate. The fetuses delivered exhibited reduced placental weight, increased frequency of cleft palate, ossification disorders in the sternum, omphalocele, and anomalous posture of forelimbs. There are no adequate and well-controlled studies in pregnant women on teratogenic effects of prednicarbate. Therefore, prednicarbate ointment 0.1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when prednicarbate ointment 0.1% is administered to a nursing woman.
Pediatric Use: Safety and effectiveness of prednicarbate ointment 0.1% in pediatric patients below the age of 10 years have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA-axis suppression when they are treated with topical corticosteroids. They are therefore also at greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing's syndrome while on treatment. Adverse effects, including striae, have been reported with inappropriate use of topical corticosteroids in pediatric patients. (See PRECAUTIONS.) HPA-axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
-
ADVERSE REACTIONS:
In controlled clinical studies, the incidence of adverse reactions associated with the use of prednicarbate ointment 0.1% was approximately 1.5%. Reported reactions including burning, pruritus, drying, scaling, cracking and pain and irritant dermatitis. The following additional local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings and especially with higher potency corticosteroids. These reactions are listed in approximate decreasing order of occurrence: folliculitis, hypertrichosis, acneform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae and miliaria.
-
OVERDOSAGE:
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (See PRECAUTIONS.)
- DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREDNICARBATE
prednicarbate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0168-0410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PREDNICARBATE (UNII: V901LV1K7D) (PREDNICARBATE - UNII:V901LV1K7D) PREDNICARBATE 1.0 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) OCTYLDODECANOL (UNII: 461N1O614Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0168-0410-15 15 g in 1 TUBE; Type 0: Not a Combination Product 03/09/2007 10/31/2023 2 NDC:0168-0410-60 60 g in 1 TUBE; Type 0: Not a Combination Product 03/09/2007 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077236 03/09/2007 12/31/2024 Labeler - Fougera Pharmaceuticals Inc. (043838424)