Label: endrate- Edetate Disodium, anhydrous injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0409-6940-03
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 11, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
- BOXED WARNING (What is this?)
-
DESCRIPTION
Endrate (Edetate Disodium Injection, USP) is a sterile, nonpyrogenic, concentrated solution of edetate disodium in water for injection which as a result of a pH adjustment with sodium hydroxide contains varying amounts of disodium and trisodium salts. After dilution, it is administered by intravenous infusion.
Each mL contains edetate disodium, anhydrous 150 mg. May contain sodium hydroxide for pH adjustment. pH is 7.0 (6.5 to 7.5).
Edetate disodium is classified as a clinical chelating agent for emergency lowering of serum calcium in hypercalcemia.
The solution contains no bacteriostat, antimicrobial agent or buffer (except for pH adjustment) and is intended only for use (after dilution) as a single-dose infusion. When smaller doses are required, the unused portion should be discarded.
Edetate Disodium, USP is chemically designated disodium (ethylenedinitrilo) tetraacetate dihydrate, a white crystalline powder soluble in water. It is also described as the disodium salt of ethylenediamine tetraacetic acid (EDTA) and has the following structural formula:
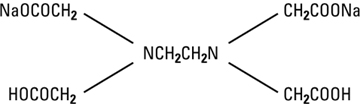
-
CLINICAL PHARMACOLOGY
Edetate Disodium Injection, USP forms chelates with the cations of calcium and many divalent and trivalent metals. Because of its affinity for calcium, edetate disodium will produce a lowering of the serum calcium level during intravenous infusion. Slow infusion over a protracted period may cause mobilization of extracirculatory calcium stores. Edetate disodiumexerts a negative inotropic effect upon the heart.
After intravenous administration, the chelate formed is excreted in the urine with 50% appearing in 1 hour and over 95% in 24 hours.
Edetate disodium likewise forms chelates with other polyvalent metals and produces increases in urinary excretion of magnesium, zinc and other trace elements. It does not form a chelate with potassium but may reduce the serum level and increase urinary loss of potassium.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
See warning statement, page 1.
Rapid intravenous infusion or attainment of high serum concentration of edetate disodium may cause a precipitous drop in the serum calcium level and many result in fatality. Toxicity appears to be dependent upon both total dosage and speed of administration. The rate of administration and dosage should not exceed that indicated in DOSAGE AND ADMINISTRATION.
Because of its irritant effect on the tissues and because of the danger of serious side effects if administered in the undiluted form, Endrate (Edetate Disodium Injection, USP) should be diluted before infusion. See DOSAGE AND ADMINISTRATION.
-
PRECAUTIONS
After the infusion of edetate disodium, the patient should remain in bed for a short time because of the possibility of postural hypotension.
The possibility of an adverse effect on myocardial contractility should be considered when administering the drug to patients with heart disease. Caution is dictated in the use of this drug in patients with limited cardiac reserve or incipient congestive failure.
Edetate Disodium Injection, USP therapy should be used with caution in patients with clinical or subclinical potassium deficiency states. In such cases it is advisable to perform serum potassium blood levels for possible hypokalemia and to monitor ECG changes.
The possibility of hypomagnesemia should be kept in mind during prolonged therapy.
Treatment with edetate disodium has been shown to cause a lowering of blood sugar and insulin requirements in patients with diabetes who are treated with insulin.
Do not use unless solution is clear and container is intact. Discard unused portion.
Laboratory Test
Renal excretory function should be assessed prior to treatment. Periodic BUN and creatinine determinations and daily urinalysis should be performed on patients receiving this drug.
Because of the possibility of inducing an electrolyte imbalance during treatment with edetate disodium, appropriate laboratory determinations and studies to evaluate the status of cardiac function should be performed. Repetition of these tests is recommended as often as clinically indicated, particularly in patients with ventricular arrhythmia and those with a history of seizure disorders or intracranial lesions. If clinical evidence suggests any disturbance of liver function during treatment, appropriate laboratory determinations should be performed and withdrawal of the drug may be required.
Drug/Laboratory Test Interactions
The oxalate method of determining serum calcium tends to give low readings in the presence of edetate disodium; modification of this method, as by acidifying the sample or use of a different method may be required for accuracy. The least interference will be noted immediately before a subsequent dose is administered.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Definitive statements cannot be made due to insufficient data and conflicting information.
Pregnancy Category C.
Animal reproduction studies have not been conducted with Edetate Disodium Injection. It is also not known whether Edetate Disodium Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Edetate Disodium Injection should be given to a pregnant woman only if clearly needed.
Geriatric Use:
Clinical studies of Endrate® did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Gastrointestinal symptoms such as nausea, vomiting and diarrhea are fairly common following administration of this drug. Transient symptoms such as circumoral paresthesia, numbness and headache and a transient drop in systolic and diastolic blood pressure may occur. Thrombophlebitis, febrile reactions, hyperuricemia, anemia, exfoliative dermatitis and other toxic skin and mucous membrane reactions have been reported.
Nephrotoxicity and damage to the reticuloendothelial system with hemorrhagic tendencies have been reported with excessive dosages.
-
OVERDOSAGE
Because of the possibility that Edetate Disodium Injection, USP may produce a precipitous drop in the serum calcium level, a source of calcium replacement suitable for intravenous administration (such as calcium gluconate) should be instantly available at the bedside before edetate disodium is administered. Extreme caution is dictated in the use of intravenous calcium in the treatment of tetany, especially in digitalized patients because the action of the drug and the replacement of calcium ions may produce a reversal of the desired digitalis effect.
-
DOSAGE AND ADMINISTRATION
Edetate Disodium Injection, USP is administered by intravenous infusion only after dilution.
For Adults: The recommended daily dosage is 50 mg/kg of body weight to a maximum dose of 3 g in 24 hours. The dose, calculated by body weight, should be diluted in 500 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP. The intravenous infusion should be regulated so that three or more hours are required for completion and the cardiac reserve of the patient is not exceeded. A suggested regimen includes five consecutive daily doses followed by two days without medication, with repeated courses as necessary to a total of 15 doses.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
Drug Interactions
Additives may be incompatible with the reconstituted (diluted) solution required for intravenous infusion. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
- HOW SUPPLIED
-
INGREDIENTS AND APPEARANCE
ENDRATE
edetate disodium, anhydrous injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-6940 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Edetate disodium, anhydrous (UNII: 8NLQ36F6MM) (Edetic acid - UNII:9G34HU7RV0) 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-6940-03 8 in 1 CASE 1 25 in 1 CONTAINER 1 20 mL in 1 AMPULE
