Label: PSEUDODINE C- triprolidine hydrochloride, pseudoephedrine hydrochloride and codeine phosphate syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 64679-809-04, 64679-809-16 - Packager: Wockhardt USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CV
Drug Label Information
Updated November 28, 2008
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 5 mL (teaspoonful) of Pseudodine™ C Cough Syrup for oral administration contains:
Triprolidine Hydrochloride, USP ............. 1.25 mg Pseudoephedrine Hydrochloride, USP..... 30 mg Codeine Phosphate, USP ........................ 10 mg Alcohol ................................................... 4.4% In addition, the following inactive ingredients are present: Artificial Fruit Flavor; Artificial Raspberry Flavor; Liquid Sugar; Menthol, USP; Methylparaben, NF; Propylene Glycol, USP; Purified Water, USP; Sodium Benzoate, NF; Sodium Citrate, USP and Sorbitol Solution, USP. It may also contain Citric Acid, USP.
Pseudodine™ C Cough Syrup has antitussive, antihistaminic and nasal decongestant effects. The components of this product have the following chemical names and structural formulas:
Codeine Phosphate, USP:
7,8-Didehydro-4,5α-epoxy-3-methoxy-17-methylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate.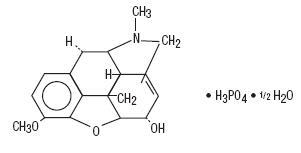
C18H21NO3•H3PO4•½ H2O M.W. 406.37
Triprolidine Hydrochloride, USP:
(E)-2-[3-(1-Pyrrolidinyl)-1-p-tolylpropenyl]pyridine monohydrochloride monohydrate.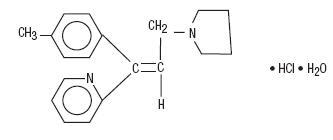
C19H22N2•HCl•H2O M.W. 332.87
Pseudoephedrine Hydrochloride, USP:
Benzenemethanol, α-[1-(methylamino)ethyl]-, [S-(R*,R*)]-, hydrochloride.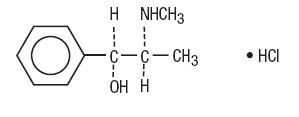
C10H15NO•HCl M.W. 201.69
-
CLINICAL PHARMACOLOGY
Codeine
Codeine probably exerts its antitussive activity by depressing the medullary (brain) cough center, thereby raising its threshold for incoming cough impulses.
Codeine is readily absorbed from the gastrointestinal tract, with a therapeutic dose reaching peak antitussive effectiveness in about 2 hours and persisting for 4 to 6 hours. Codeine is rapidly distributed from blood to body tissues and taken up preferentially by parenchymatous organs such as liver, spleen and kidney. It passes the blood brain barrier and is found in fetal tissue and breast milk.
The drug is not bound by plasma proteins nor is it accumulated in body tissues. Codeine is metabolized in the liver to morphine and norcodeine, each representing about 10 percent of the administered codeine dose. About 90 percent of the dose is excreted within 24 hours, primarily through the kidneys. Urinary excretion products are free and glucuronide-conjugated codeine (about 70%), free and conjugated norcodeine (about 10%), free and conjugated morphine (about 10%), normorphine (under 4%) and hydrocodone (<1%). The remainder of the dose appears in the feces.
Triprolidine
Antihistamines such as triprolidine hydrochloride act as antagonists of the H1 histamine receptor. Consequently, they prevent histamine from eliciting typical immediate hypersensitivity responses in the nose, eyes, lungs and skin.
Animal distribution studies have shown localization of triprolidine in lung, spleen and kidney tissue. Liver microsome studies have revealed the presence of several metabolites with an oxidized product of the toluene methyl group predominating.
Pseudoephedrine
Pseudoephedrine acts as an indirect sympathomimetic agent by stimulating sympathetic (adrenergic) nerve endings to release norepinephrine. Norepinephrine in turn stimulates alpha and beta receptors throughout the body. The action of pseudoephedrine hydrochloride is apparently more specific for the blood vessels of the upper respiratory tract and less specific for the blood vessels of the systemic circulation. The vasoconstriction elicited at these sites results in the shrinkage of swollen tissues in the sinuses and nasal passages.
Pseudoephedrine is rapidly and almost completely absorbed from the gastrointestinal tract. Considerable variation in half-life has been observed (from about 4½ to 10 hours), which is attributed to individual differences in absorption and excretion. Excretion rates are also altered by urine pH, increasing with acidification and decreasing with alkalinization. As a result, mean half-life falls to about 4 hours at pH 5 and increases to 12 to 13 hours at pH 8.
After administration of a 60 mg tablet, 87 to 96% of the pseudoephedrine is cleared from the body within 24 hours. The drug is distributed to body tissues and fluids, including fetal tissue, breast milk and the central nervous system (CNS). About 55 to 75% of an administered dose is excreted unchanged in the urine; the remainder is apparently metabolized in the liver to inactive compounds by N-demethylation, parahydroxylation and oxidative deamination.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Pseudodine™ C Cough Syrup is contraindicated under the following conditions:
Use in Lower Respiratory Disease
Antihistamines should not be used to treat lower respiratory tract symptoms, including asthma.
Hypersensitivity to
(1) codeine phosphate or other narcotics; (2) triprolidine hydrochloride or other antihistamines of similar chemical structure; or (3) sympathomimetic amines, including pseudoephedrine.
Sympathomimetic amines are contraindicated in patients with severe hypertension, severe coronary artery disease and in patients on monoamine oxidase (MAO) inhibitor therapy (see PRECAUTIONS, Drug Interactions).
-
WARNINGS
Pseudodine™ C Cough Syrup should be used with considerable caution in patients with increased intraocular pressure (narrow angle glaucoma), stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, bladder neck obstruction, hypertension, diabetes mellitus, ischemic heart disease, and hyperthyroidism.
In the presence of head injury or other intracranial lesions, the respiratory depressant effects of codeine and other narcotics may be markedly enhanced, as well as their capacity for elevating cerebrospinal fluid pressure.
Narcotics also produce other CNS depressant effects, such as drowsiness, that may further obscure the clinical course of patients with head injuries.
Codeine or other narcotics may obscure signs on which to judge the diagnosis or clinical course of patients with acute abdominal conditions.
-
PRECAUTIONS
General
Pseudodine™ C Cough Syrup should be prescribed with caution for certain special-risk patients, such as the elderly or debilitated, and for those with severe impairment of renal or hepatic function, gallbladder disease or gallstones, respiratory impairment, cardiac arrhythmias, history of bronchial asthma, prostatic hypertrophy or urethral stricture, and in patients known to be taking other antitussive, antihistamine or decongestant medications. Patients' self-medication habits should be investigated to determine their use of such medications. Pseudodine™ C Cough Syrup is intended for short-term use only.
Ultra-rapid Metabolizers of Codeine
Some individuals may be ultra-rapid metabolizers due to a specific CYP2D6*2×2 genotype. These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regiments, individuals who are ultra-rapid metabolizers may experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing.
The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1 to 10% in Caucasians, 3% in African Americans, and 16 to 28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups.
When physicians prescribe codeine-containing drugs, they should choose the lowest effective dose for the shortest period of time and should inform their patients about these risks and the signs of morphine overdose. (See PRECAUTIONS-Nursing Mothers)
Information for Patients
- Patients should be warned about engaging in activities requiring mental alertness such as driving a car, operating dangerous machinery or hazardous appliances.
- Patients with a history of glaucoma, peptic ulcer, urinary retention or pregnancy should be cautioned before starting Pseudodine™ C Cough Syrup.
- Patients should be told not to take alcohol, sleeping pills, sedatives or tranquilizers while taking Pseudodine™ C Cough Syrup.
- Antihistamines may cause dizziness, drowsiness, dry mouth, blurred vision, weakness, nausea, headache or nervousness in some patients.
- Patients should be told to store this medicine in a tightly closed container in a dry, cool place away from heat or direct sunlight and out of the reach of children.
- Nursing Mothers - refer to following section titled "Nursing Mothers".
Pseudodine™ C Cough Syrup should not be used by persons intolerant to sympathomimetics used for the relief of nasal or sinus congestion. Such drugs include ephedrine, epinephrine, phenylephrine and phenylpropanolamine. Symptoms of intolerance include drowsiness, dizziness, weakness, difficulty in breathing, tenseness, muscle tremors or palpitations.
Codeine may be habit-forming when used over long periods or in high doses. Patients should take the drug only for as long, in the amounts, and as frequently as prescribed.
Caution patients that some people have a variation in a liver enzyme and change codeine into morphine more rapidly and completely than other people. These people are ultra-rapid metabolizers and are more likely to have higher-than-normal levels of morphine in their blood after taking codeine which can result in overdose symptoms such as extreme sleepiness, confusion, or shallow breathing. In most cases, it is unknown if someone is an ultra-rapid codeine metabolizer.
Nursing mothers taking codeine can also have higher morphine levels in their breast milk if they are ultra-rapid metabolizers. These higher levels of morphine in breast milk may lead to life-threatening or fatal side effects in nursing babies. Instruct nursing mothers to watch for signs of morphine toxicity in their infants including increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to the baby's doctor immediately if they notice these signs and, if they cannot reach the doctor right away, to take the baby to an emergency room or call 911 (or local emergency services).
Drug Interactions
Pseudodine™ C Cough Syrup may enhance the effects of:
- Monoamine oxidase (MAO) inhibitors;
- other narcotic analgesics, alcohol, general anesthetics, tranquilizers, sedative-hypnotics, surgical skeletal muscle relaxants, or other CNS depressants, by causing increased CNS depression.
Pseudodine™ C Cough Syrup may diminish the antihypertensive effects of guanethidine, bethanidine, methyldopa and reserpine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether the components of Pseudodine™ C Cough Syrup have a potential for carcinogenesis, mutagenesis or impairment of fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Pseudodine™ C Cough Syrup. It is also not known whether Pseudodine™ C Cough Syrup can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pseudodine™ C Cough Syrup should be given to a pregnant woman only if clearly needed.
Teratology studies have been conducted with the three ingredients of Pseudodine™ C Cough Syrup. Pseudoephedrine studies were conducted in rats at doses up to 150 times the human dose; triprolidine was studied in rats and rabbits at doses up to 125 times the human dose, and codeine studies were conducted in rats and rabbits at doses up to 150 times the human dose. No evidence of teratogenic harm to the fetus was revealed in any of these studies. However, overt signs of toxicity were observed in the dams which received pseudoephedrine. This was reflected in reduced average weight and length and rate of skeletal ossification in their fetuses.
Nursing Mothers
The components of Pseudodine™ C Cough Syrup are excreted in breast milk in small amounts, but the significance of their effects on nursing infants is not known. Because of the potential for serious adverse reactions in nursing infants from maternal ingestion of Pseudodine™ C Cough Syrup, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose-dependent. Despite the common use of codeine products to manage postpartum pain, reports of adverse events in infants are rare. However, some women are ultra-rapid metabolizers of codeine. These women achieve higher-than-expected serum levels of codeine's active metabolite, morphine, leading to higher-than-expected levels of morphine in breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, maternal use of codeine can potentially lead to serious adverse reactions, including death, in nursing infants.
The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1 to 10% in Caucasians, 3% in African Americans, and 16 to 28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups.
The risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and baby. Caution should be excercised when codeine is administered to a nursing woman. If a codeine containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Mothers using codeine should be informed about when to seek immediate medical care and how to identify the signs and symptoms of neonatal toxicity, such as drowsiness or sedation, difficulty breastfeeding, breathing difficulties, and decreased tone, in their baby. Nursing mothers who are ultra-rapid metabolizers may also experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. Prescribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine during breastfeeding. (See PRECAUTIONS–General-Ultra-rapid Metabolizers of Codeine)
Pediatric Use
As in adults, the combination of an antihistamine, sympathomimetic amine and codeine can elicit either mild stimulation or mild sedation in pediatric patients. In pediatric patients particularly, the ingredients in this drug product in overdosage may produce hallucinations, convulsions and death. Symptoms of toxicity in pediatric patients may include fixed dilated pupils, flushed face, dry mouth, fever, excitation, hallucinations, ataxia, incoordination, athetosis, tonic clonic convulsions and postictal depression. (See CONTRAINDICATIONS and OVERDOSAGE sections.)
-
ADVERSE REACTIONS
(The most frequent adverse reactions are underlined.)
General: Dryness of mouth, dryness of nose, dryness of throat, urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration and chills.
Cardiovascular System: Hypotension, headache, palpitations, tachycardia, extrasystoles.
Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, anxiety, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions, CNS depression, hallucination.
G.I. System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
G.U. System: Urinary frequency, difficult urination, urinary retention, early menses.
Respiratory System: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness, respiratory depression.
-
DRUG ABUSE AND DEPENDENCE
Like other medications containing a narcotic, Pseudodine™ C Cough Syrup is controlled by the Drug Enforcement Administration. It is classified under Schedule V.
Codeine can produce drug dependence of the morphine type, and therefore it has a potential for being abused. Psychic dependence, physical dependence and tolerance may develop on repeated administration.
The dependence liability of codeine has been found to be too small to permit a full definition of its characteristics. Studies indicate that addiction to codeine is extremely uncommon and requires very high parenteral doses.
When dependence on codeine occurs at therapeutic doses, it appears to require from one to two months to develop, and withdrawal symptoms are mild. Most patients on long-term oral codeine therapy show no signs of physical dependence upon abrupt withdrawal.
-
OVERDOSAGE
Since Pseudodine™ C Cough Syrup is comprised of three pharmacologically different compounds, it is difficult to predict the exact manifestation of symptoms in a given individual. Reaction to an overdosage of Pseudodine™ C Cough Syrup may vary from CNS depression to stimulation. A detailed description of symptoms which are likely to appear after ingestion of an excess of the individual components follows:
Overdosage with codeine can cause transient euphoria, drowsiness, dizziness, weariness, diminution of sensitivity, loss of sensation, vomiting, transient excitement in children, and occasionally in adult women, miosis progressing to nonreactive pinpoint pupils, itching sometimes with skin rashes and urticaria and clammy skin with mottled cyanosis. In more severe cases, muscular relaxation with depressed or absent superficial and deep reflexes and a positive Babinski sign may appear. Marked slowing of the respiratory rate with inadequate pulmonary ventilation and consequent cyanosis may occur. Terminal signs include shock, pulmonary edema, hypostatic or aspiration pneumonia and respiratory arrest, with death occurring within 6 to 12 hours following ingestion.
Overdoses of antihistamines may cause hallucinations, convulsions, or possibly death, especially in infants and children. Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
Overdosage with triprolidine may produce reactions varying from depression to stimulation of the Central Nervous System (CNS); the latter is particularly likely in children. Atropine-like signs and symptoms (dry mouth, fixed dilated pupils, flushing, tachycardia, hallucinations, convulsions, urinary retention, cardiac arrhythmias and coma) may occur.
Overdosage with pseudoephedrine can cause excessive CNS stimulation resulting in excitement, nervousness, anxiety, tremor, restlessness and insomnia. Other effects include tachycardia, hypertension, pallor, mydriasis, hyperglycemia and urinary retention. Severe overdosage may cause tachypnea or hyperpnea, hallucinations, convulsions, or delirium, but in some individuals there may be CNS depression with somnolence, stupor or respiratory depression. Arrhythmias (including ventricular fibrillation) may lead to hypotension and circulatory collapse. Severe hypokalemia can occur, probably due to compartmental shift rather than depletion of potassium. No organ damage or significant metabolic derangement is associated with pseudoephedrine overdosage.
The toxic plasma concentration of codeine is not known with certainty. Experimental production of mild to moderate CNS depression in healthy, nontolerant subjects occurs at plasma concentrations of 0.5 to 1.9 mcg/mL when codeine is given by intravenous infusion. The single lethal dose of codeine in adults is estimated to be from 0.5 to 1 gram. It is also estimated that 5 mg/kg could be fatal in children.
The LD50 (single, oral dose) of triprolidine is 163 to 308 mg/kg in the mouse (depending upon strain) and 840 mg/kg in the rat.
Insufficient data are available to estimate the toxic and lethal doses of triprolidine in humans. No reports of acute poisoning with triprolidine have appeared.
The LD50 (single, oral dose) of pseudoephedrine is 726 mg/kg in the mouse, 2206 mg/kg in the rat and 1177 mg/kg in the rabbit. The toxic and lethal concentrations in human biologic fluids are not known. Excretion rates increase with urine acidification and decrease with alkalinization. Few reports of toxicity due to pseudoephedrine have been published and no case of fatal overdosage is known.
Therapy, if instituted within 4 hours of overdosage, is aimed at reducing further absorption of the drug. In the conscious patient, vomiting should be induced even though it may have occurred spontaneously. If vomiting cannot be induced, gastric lavage is indicated. Adequate precautions must be taken to protect against aspiration, especially in infants and children. Charcoal slurry or other suitable agents should be instilled into the stomach after vomiting or lavage. Saline cathartics or milk of magnesia may be of additional benefit.
In the unconscious patient, the airway should be secured with a cuffed endotracheal tube before attempting to evacuate the gastric contents. Intensive supportive and nursing care is indicated, as for any comatose patient.
If breathing is significantly impaired, maintenance of an adequate airway and mechanical support of respiration is the most effective means of providing adequate oxygenation.
Hypotension is an early sign of impending cardiovascular collapse and should be treated vigorously. Do not use CNS stimulants. Convulsions should be controlled by careful administration of diazepam or short-acting barbiturate, repeated as necessary. Physostigmine may be also considered for use in controlling centrally mediated convulsions.
Ice packs and cooling sponge baths, not alcohol, can aid in reducing the fever commonly seen in children.
For codeine, continuous stimulation that arouses, but does not exhaust, the patient is useful in preventing coma. Continuous or intermittent oxygen therapy is usually indicated, while naloxone is useful as a codeine antidote. Close nursing care is essential.
Saline cathartics, such as milk of magnesia, help to dilute the concentration of the drugs in the bowel by drawing water into the gut, thereby hastening drug elimination.
Adrenergic receptor blocking agents are antidotes to pseudoephedrine. In practice, the most useful is the beta-blocker propranolol, which is indicated when there are signs of cardiac toxicity.
There are no specific antidotes to triprolidine. Histamine should not be given.
Pseudoephedrine and codeine are theoretically dialyzable, but the procedures have not been clinically established.
In severe cases of overdosage, it is essential to monitor both the heart (by electrocardiograph) and plasma electrolytes and to give intravenous potassium as indicated by these continuous controls. Vasopressors may be used to treat hypotension, and excessive CNS stimulation may be counteracted with parenteral diazepam. Stimulants should not be used.
-
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND RESPONSE OF THE PATIENT.
Usual Dose: Teaspoonfuls (5 mL) Adults and children 12 years and older 2 teaspoonfuls (10 mL) every 4 to 6 hours, not to exceed 8 teaspoonfuls (40 mL) in 24 hours. Children 6 to under 12 years 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 4 teaspoonfuls (20 mL) in 24 hours. Children 2 to under 6 years ½ teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 2 teaspoonfuls (10 mL) in 24 hours. - HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
PSEUDODINE C
triprolidine hydrochloride, pseudoephedrine hydrochloride and codeine phosphate syrupProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64679-809 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Triprolidine Hydrochloride (UNII: YAN7R5L890) (Triprolidine - UNII:2L8T9S52QM) 1.25 mg in 5 mL Pseudoephedrine Hydrochloride (UNII: 6V9V2RYJ8N) (Pseudoephedrine - UNII:7CUC9DDI9F) 30 mg in 5 mL Codeine Phosphate (UNII: GSL05Y1MN6) (Codeine - UNII:Q830PW7520) 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Artificial Fruit Flavor () Artificial Raspberry Flavor () Sugar (UNII: 8M707QY5GH) Menthol () Methylparaben (UNII: A2I8C7HI9T) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Sodium Benzoate (UNII: OJ245FE5EU) Sodium Citrate (UNII: 1Q73Q2JULR) Sorbitol (UNII: 506T60A25R) Citric Acid (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64679-809-04 118 mL in 1 BOTTLE 2 NDC:64679-809-16 473 mL in 1 BOTTLE Labeler - Wockhardt USA, Inc.
