Label: BENZNIDAZOLE tablet
- NDC Code(s): 0642-7463-12, 0642-7464-10
- Packager: Exeltis USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BENZNIDAZOLE TABLETS safely and effectively. See full prescribing information for BENZNIDAZOLE TABLETS.
BENZNIDAZOLE tablets, for oral use
Initial U.S. Approval: 2017INDICATIONS AND USAGE
Benznidazole Tablets, a nitroimidazole antimicrobial, is indicated in pediatric patients 2 to 12 years of age for the treatment of Chagas disease (American trypanosomiasis), caused by Trypanosoma cruzi ( 1).
This indication is approved under accelerated approval based on the number of treated patients who became Immunoglobulin G (IgG) antibody negative against the recombinant antigens of T. cruzi. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials ( 1, 14).
DOSAGE AND ADMINISTRATION
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Potential Risk for Genotoxicity and Carcinogenicity ( 5.1).
- Embryo-Fetal Toxicity: Can cause fetal harm. Pregnancy testing is recommended for females of reproductive potential. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception ( 2.3, 5.2, 8.1, 8.3).
- Hypersensitivity skin reactions have been reported with benznidazole. In case of skin reactions, presenting with additional symptoms of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is recommended ( 5.3).
- Treatment with Benznidazole Tablets can potentially cause paresthesia or symptoms of peripheral neuropathy. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended ( 5.4).
- There have been hematological manifestations of bone marrow depression, such as neutropenia, thrombocytopenia, anemia, and leukopenia ( 5.5).
ADVERSE REACTIONS
Most common adverse reactions observed were abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Exeltis USA, Inc. at 1-877-324-9349 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage in Pediatric Patients (2 to 12 Years of Age)
2.3 Assessment Prior to Initiating Benznidazole Tablets
2.4 Preparation of Slurry as an Alternative Method of Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Disulfiram
4.3 Patients with Cockayne Syndrome
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Genotoxicity and Carcinogenicity
5.2 Embryo-Fetal Toxicity
5.3 Hypersensitivity Skin Reactions
5.4 Central and Peripheral Nervous System Effects
5.5 Hematological Manifestations of Bone Marrow Depression
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Disulfiram
7.2 Alcohol and Products Containing Propylene Glycol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Benznidazole Tablets are indicated in pediatric patients 2 to 12 years of age for the treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi.
This indication is approved under accelerated approval based on the number of treated patients who became Immunoglobulin G (IgG) antibody negative against the recombinant antigens of T. cruzi [see Clinical Studies ( 14)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Benznidazole Tablets (12.5 mg and 100 mg) are for oral use and may be taken with or without food [see Clinical Pharmacology ( 12.3)] .
- Benznidazole Tablets are dosed by body weight (kg) [see Dosage and Administration ( 2.2)] .
- Benznidazole Tablets 100 mg are functionally scored tablets which can be split into one-half (50 mg) or one-quarter (25 mg) at the scored lines to provide doses less than 100 mg [see Instructions for Use] .
- Benznidazole Tablets 12.5 mg and 100 mg can be made into slurry as an alternative method of administration [see Dosage and Administration ( 2.4)] .
2.2 Recommended Dosage in Pediatric Patients (2 to 12 Years of Age)
The total daily dose for pediatric patients 2 to 12 years of age is 5 mg/kg to 8 mg/kg orally administered in two divided doses separated by approximately 12 hours, for a duration of 60 days (see Table 1).
Table 1: Recommended Dosage of Benznidazole Tablets in Pediatric Patients (2 to 12 Years of Age) Body Weight Range (kg)
Dose (mg)
Number of Benznidazole Tablets
12.5 mgNumber of Benznidazole Tablets
100mgDuration and Frequency of Therapy
Less than 15 kg
50 mg
4 tablets
½ tablet
Administered twice daily approximately 12 hours apart for 60 days.
15 kg to less than 20 kg
62.5 mg
5 tablets
20 kg to less than 30 kg
75 mg
6 tablets
¾ tablet
30 kg to less than 40 kg
100 mg
1 tablet
40 kg to less than 60 kg
150 mg
1 ½ tablets
Greater than or equal to 60 kg
200 mg
2 tablets
2.3 Assessment Prior to Initiating Benznidazole Tablets
Obtain a pregnancy test in females of reproductive potential prior to therapy with Benznidazole Tablets [see Use is Specific Populations ( 8.3)] .
2.4 Preparation of Slurry as an Alternative Method of Administration
A. Preparation of Slurry Using Benznidazole Tablets 12.5 mg for the Pediatric Population with Body Weight Less Than 30 kg
Benznidazole Tablets 12.5 mg may be made into slurry in a specified volume of water for the pediatric population with body weight less than 30 kg (see Table 2). The 12.5 mg tablet slurry is prepared by the following method:
Table 2: Preparation and Administration of Slurry Using Benznidazole Tablets 12.5 mg for the Pediatric Population with Body Weight of Less than 30 kg - Place the prescribed dose of Benznidazole Tablets 12.5 mg into a cup.
- Add the specified volume of water per number of 12.5 mg tablets as shown below.
Body Weight Range (kg)
Dose
(mg)Number of Benznidazole Tablets 12.5 mg
Quantity of Water for Preparing the Slurry
Less than 15 kg
50 mg
4 tablets
40 mL
15 kg to less than 20 kg
62.5 mg
5 tablets
50 mL
20 kg to less than 30 kg
75 mg
6 tablets
60 mL
- Allow the tablets to disintegrate in the cup over a period of approximately 1-2 minutes.
- Shake the contents of the cup gently to mix.
- Drink the contents of the cup (slurry of tablets with water) immediately.
- Rinse the cup with an additional 10 mL of water and drink the whole amount.
B. Preparation of Slurry Using Benznidazole Tablets 100 mg for the Pediatric Population with Body Weight (30 kg or greater)
Benznidazole Tablets 100 mg may be made into a slurry in a specified volume of water for the pediatric population with body weight of 30 kg or greater (see Table 3). The 100 mg tablet slurry is prepared as follows:
Table 3: Preparation and Administration of Slurry Using Benznidazole Tablets 100 mg for the Pediatric Population with Body Weight 30 kg or greater - Place the prescribed dose of Benznidazole Tablets 100 mg tablets into a cup.
- Add the specified volume of water per number of 100 mg tablets as shown below.
Body Weight Range (kg)
Dose
(mg)Number of Benznidazole Tablets 100 mg
Quantity of Water for Preparing the Slurry
30 kg to less than 40 kg
100 mg
1 tablet
80 mL
40 kg to less than 60 kg
150 mg
1 ½ tablets
120 mL
Greater than or equal to 60 kg
200 mg
2 tablets
160 mL
- Allow the tablet(s) to disintegrate in the cup over a period of approximately 1- 2 minutes.
- Shake the contents of the cup gently to mix.
- Drink the contents of the cup (slurry of tablet(s) with water) immediately.
- Rinse the cup by adding 80 mL of water and drink the whole amount. Repeat this rinse with 80 mL of water and drink again.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Benznidazole Tablets are contraindicated in patients with a history of hypersensitivity reaction to benznidazole or other nitroimidazole derivatives. Reactions have included severe skin and soft tissue reactions [see Adverse Reactions ( 6.1)] .
4.2 Disulfiram
Benznidazole Tablets are contraindicated in patients who have taken disulfiram within the last two weeks. Psychotic reactions may occur in patients who are using benznidazole and disulfiram concurrently [see Drug Interactions ( 7.1)] .
4.3 Patients with Cockayne Syndrome
Benznidazole Tablets are contraindicated in patients with Cockayne syndrome. Severe irreversible hepatotoxicity/acute liver failure with fatal outcomes have been reported after initiation of metronidazole, another nitroimidazole drug, structurally related to benznidazole in patients with Cockayne syndrome [see Adverse Reactions ( 6.2)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Genotoxicity and Carcinogenicity
Genotoxicity
Genotoxicity of benznidazole has been demonstrated in humans, in vitro in several bacterial species and mammalian cell systems, and in vivo in rodents [see Nonclinical Toxicology ( 13.1)] .
A study evaluating the cytogenetic effect of benznidazole in pediatric patients ranging from 11 months to 11 years of age (the safety and effectiveness of Benznidazole Tablets in patients less than 2 years old has not been established) with Chagas disease demonstrated a two-fold increase in chromosomal aberrations. In pediatric patients with Chagas disease who were treated with benznidazole, the median incidence of micronucleated interphase lymphocytes in 20 patients increased 2-fold compared to pre-dose values. In the same study, the mean incidence of chromosomal aberrations in 10 patients also increased 2-fold compared to pre-dose values.
Carcinogenicity
Carcinogenicity has been observed in mice and rats treated chronically with nitroimidazole agents which are structurally similar to benznidazole. Similar data have not been reported for benznidazole [see Nonclinical Toxicology ( 13.1)] . It is not known whether benznidazole is associated with carcinogenicity in humans.
5.2 Embryo-Fetal Toxicity
Based on findings from animal studies, Benznidazole Tablets can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, benznidazole administered orally to pregnant rats and rabbits during organogenesis was associated with fetal malformations at doses approximately 1-3 times the maximum recommended human dose (MRHD) in rats (anasarca, anophthalmia, and/or microphthalmia) and doses approximately 0.3-1 times the MRHD in rabbits (ventricular septal defect). In rats, reduced maternal weights and smaller litter sizes occurred at a dose approximately 3 times the MRHD. In rabbits, reduced maternal weight gain, and abortions in 2/20 females occurred at a dose approximately equal to the MHRD [see Use in Specific Populations ( 8.1)] . Advise pregnant women of the potential risk to a fetus. Pregnancy testing is recommended for females of reproductive potential [see Dosage and Administration ( 2.3)] . Advise females of reproductive potential to use effective contraception during treatment with Benznidazole Tablets and for 5 days after the last dose [see Use in Specific Populations ( 8.1, 8.3) and Clinical Pharmacology ( 12.3)] .
5.3 Hypersensitivity Skin Reactions
Serious skin and subcutaneous disorders including acute generalized exanthematous pustulosis (AGEP), toxic epidermal necrolysis (TEN), erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported with benznidazole. Discontinue treatment at the first evidence of these serious cutaneous reactions [see Adverse Reactions ( 6.2)] .
Extensive skin reactions, such as rash (maculopapular, pruritic macules, eczema, pustules, erythematous, generalized, and allergic dermatitis, exfoliative dermatitis) have also been reported. Most cases occurred after approximately 10 days of treatment with benznidazole. Most rashes resolved with treatment discontinuation.
In case of skin reactions presenting with additional symptoms or signs of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is recommended.
5.4 Central and Peripheral Nervous System Effects
Treatment with Benznidazole Tablets can cause paresthesia or symptoms of peripheral neuropathy that may take several months to resolve. Headache and dizziness have been reported. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended. In most cases, symptoms occur late in the course of treatment.
5.5 Hematological Manifestations of Bone Marrow Depression
There have been reports of hematological manifestations of bone marrow depression, such as neutropenia, thrombocytopenia, anemia and leukopenia, which resolved after treatment discontinuation [see Adverse Reactions ( 6.1)] . Patients with hematological manifestations of bone marrow depression must take Benznidazole Tablets only under strict medical supervision. Monitor complete blood count. Total and differential leukocyte counts are recommended before, during and after therapy.
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
- Potential for Genotoxicity, Carcinogenicity, and Mutagenicity [see Warnings and Precautions ( 5.1)]
- Hypersensitivity Skin Reactions [see Warnings and Precautions ( 5.3)]
- Central and Peripheral Nervous System Effects [see Warnings and Precautions ( 5.4)]
- Hematological Manifestations of Bone Marrow Depression [see Warnings and Precautions ( 5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Benznidazole was evaluated in two randomized, double-blind, placebo-controlled trials (Trial 1 1 and Trial 2 2) and one uncontrolled trial (Trial 3 3).
Trial 1 was conducted in pediatric patients 6 to 12 years of age with chronic indeterminate Chagas disease in Argentina. The chronic indeterminate form includes patients with serologic evidence of T. cruzi infection without symptoms of cardiac or gastrointestinal disease. A total of 106 patients were randomized to receive either benznidazole (5 mg/kg/day twice daily for 60 days; N= 55) or placebo (N=51) and followed for 4 years.
Trial 2 was conducted in pediatric patients 7 to 12 years of age with chronic indeterminate Chagas disease in Brazil. A total of 129 patients were randomized to receive either benznidazole (7.5 mg/kg/day twice daily for 60 days; N = 64) or placebo (N = 65) and followed for 3 years.
Trial 3 was an uncontrolled study in pediatric patients 2 to 12 years of age with chronic indeterminate Chagas disease. A total of 37 pediatric patients with Chagas disease were enrolled in this safety and pharmacokinetics study. Patients were treated with benznidazole 5 to 8 mg/kg/day twice daily for 60 days.
Adverse Reactions Leading to Discontinuation
In Trial 1, benznidazole was discontinued due to an adverse reaction in 5/55 (9%) patients. Some patients had more than one adverse reaction resulting in treatment discontinuation. The adverse reactions included abdominal pain, nausea, vomiting, rash, decreased appetite, headache, and transaminases increased.
Common Adverse Reactions in Pediatric Patients
The most frequently reported adverse reactions in pediatric patients treated with benznidazole in Trial 1 were abdominal pain (25%), rash (16%), decreased weight (13%), and headache (7%). Table 4 lists adverse reactions occurring at a rate of 1% or greater in pediatric patients with Chagas disease aged 6 to 12 years of age in Trial 1.
Table 4: Adverse Reactions Occurring in Pediatric Patients with Chagas Disease aged 6 to 12 Years in Trial 1 Body System
Adverse Reaction
Benznidazole
(N=55)
N (%)Placebo
(N=51)
N (%)Gastrointestinal
Abdominal pain
14 (25)
4 (8)
Weight decreased
7 (13)
1 (2)
Nausea
3 (5)
1 (2)
Vomiting
3 (5)
0
Diarrhea
2 (4)
0
Decreased appetite
3 (5)
0
Skin and subcutaneous tissue
Rash
9 (16)
0
Metabolism/Laboratory
Transaminases increased
3 (5)
0
Nervous system Disorders
Dizziness
2 (4)
2 (4)
Peripheral neuropathy
1 (2)
0
Tremor
1 (2)
0
In Trial 2, skin lesions were reported in 7 of 64 (11%) pediatric patients treated with benznidazole and in 2 of 65 patients receiving placebo. Adverse reactions reported in fewer than 5% of benznidazole-treated patients included nausea, anorexia, headache, abdominal pain and arthralgia.
In a subset of 19 pediatric patients 2 to 6 years of age treated with benznidazole in Trial 3, 6 patients (32%) had the following adverse reactions: rash, leukopenia, urticaria, eosinophilia, decreased appetite, and neutropenia. These adverse reactions were similar to those observed in the overall population of 37 patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the use of other formulations of benznidazole outside of the United States, or other nitroimidazole agents . Because these reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Other Formulations of Benznidazole:
Table 5: Adverse Reactions Reported in the Published Literature Body System
Adverse Reactions
Dermatological
- Maculo-papular cutaneous eruptions
- Erythematous plaques
- Rash, generalized
- Rash, erythematous
- Pruritic rash
- Blistering eruptions
- Peeling skin
- Exfoliative dermatitis
- Toxic epidermal necrolysis
- AGEP
- Erythema multiforme
- Drug reaction with eosinophilia and systemic symptoms (DRESS)
Neurological (central and peripheral nervous system)
- Paresthesia
- Hypoesthesia
- Headaches
- Insomnia
- Convulsions
- Inability to concentrate
- Amnesia, temporary
- Disorientation, temporary
Gastrointestinal
- Epigastric pain
- Dry mouth
- Ageusia
Hepatobiliary disorders
- Hepatitis
- Toxic hepatitis
Skeletal Muscle
- Myalgia
- Musculoskeletal pain
- Migratory arthritis
General / Constitutional Symptoms
- Fever
- Asthenia
- Fatigue
Lymphatic
- Generalized edema
- Eyelid edema
- Edema in the extremities
- Lymphadenopathy
Bone Marrow
- Thrombocytopenia
- Granulocytopenia
- Agranulocytosis
Metabolism / Laboratory
- Elevation of alkaline phosphatase
- Elevation of bilirubin
Metronidazole, Another Nitroimidazole Agent, Structurally Related to Benznidazole
Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, another nitroimidazole agent structurally related to benznidazole, have been reported in patients with Cockayne syndrome (latency from drug start to signs of liver failure as short as 2 days) [see Contraindications ( 4.3)] .
-
7 DRUG INTERACTIONS
7.1 Disulfiram
Psychotic reactions have been reported in patients who are concurrently taking disulfiram and nitroimidazole agents (structurally related to benznidazole, but not with benznidazole). Benznidazole Tablets should not be given to patients who have taken disulfiram within the last two weeks [see Contraindications ( 4.2)] .
7.2 Alcohol and Products Containing Propylene Glycol
In vitro studies showed that benznidazole, at concentrations from 0.03 μM to 100 μM, does not inhibit the enzymatic activity of human alcohol dehydrogenase (ALDH). Benznidazole Tablets are not expected to cause alcohol aversion or a disulfiram-like reaction as a result of ethanol ingestion.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, Benznidazole Tablets may cause fetal harm when administered to a pregnant woman. Published postmarketing reports on benznidazole use during pregnancy are insufficient to inform a drug-associated risk of adverse pregnancy-related outcomes. There are risks to the fetus associated with Chagas Disease (see Clinical Considerations) . In embryo-fetal development studies, benznidazole administered orally to pregnant rats and rabbits during organogenesis was associated with fetal malformations at doses approximately 1-3 times the MRHD in rats (anasarca, anophthalmia, and/or microphthalmia) and doses approximately 0.3-1.0 times the MRHD in rabbits (ventricular septal defect). In pregnant rats administered benznidazole during gestation and through lactation, effects in first generation offspring included reduced fertility in individual males and reduced numbers of live embryos and fetuses in pregnant females at a maternal dose equivalent to approximately 1.5 times the MRHD (see Data) . Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk
Published data from case-control and observational studies on chronic Chagas disease during pregnancy are inconsistent in their findings. Some studies showed an increased risk of pregnancy loss, prematurity and neonatal mortality in pregnant women who have chronic Chagas disease while other studies did not demonstrate these findings. Chronic Chagas disease is usually not life-threatening. Since pregnancy findings are inconsistent, treatment of chronic Chagas disease during pregnancy is not recommended due to risk of embryo-fetal toxicity from Benznidazole Tablets.
Acute symptomatic Chagas disease is rare in pregnant women; however, symptoms may be serious or life-threatening. There have been reports of pregnant women with life-threatening symptoms associated with acute Chagas disease who were treated with benznidazole. If a pregnant woman presents with acute symptomatic Chagas disease, the risks versus benefits of treatment with Benznidazole Tablets to the mother and the fetus should be evaluated on a case-by-case basis.
Data
Animal Data
In an embryo-fetal toxicity study in pregnant rats, benznidazole was administered in oral doses of 15, 50, and 150 mg/kg/day during organogenesis (days 6-17 of gestation), and the high dose was associated with maternal weight loss, reduced fetal weights, and smaller litter sizes. Benznidazole was also associated with a low incidence of fetal malformations including anasarca in one fetus at a dose of 50 mg/kg/day and anasarca and eye abnormalities (anophthalmia and microphthalmia) in 5 fetuses in 5 litters at a high dose of 150 mg/kg/day (approximately equivalent to 1 and 3 times, respectively, the MRHD based on whole body surface area comparisons). No maternal toxicity was observed at a benznidazole dose of 50 mg/kg/day (approximately equal to the MRHD based on body surface area comparison), and no fetal toxicity was observed at a dose of 15 mg/kg/day (approximately equivalent to 0.3 times the MRHD based on whole body surface area comparison).
In an embryo-fetal study in pregnant rabbits benznidazole administered by oral (gavage) in doses of 2.5, 7.5, and 25 mg/kg/day during organogenesis (days 6 to 19 of gestation) was associated with maternal toxicity at the high dose, including reduced weight gain and food consumption and abortions in 2/20 females. Benznidazole was also associated with a low incidence of fetal abnormalities including ventricular septal defect in 2 fetuses in 2 litters at a dose of 7.5 mg/kg/day and in 1 fetus at a dose of 25 mg/kg/day (approximately equivalent to 0.3 and 1 times respectively the MRHD based on whole body surface area comparison).The benznidazole doses that were not associated with maternal and fetal toxicity in this study were 7.5 and 2.5 mg/kg/day respectively, which are respectively equivalent to approximately 0.3 and 0.1 times the MRHD based on whole body surface area comparison.
In a pre-postnatal study in rats, first generation (F 1) pups born to dams administered 15, 50, and 75 mg/kg/day benznidazole from day 6 of gestation to day 20 of lactation demonstrated normal pre-weaning behavior, physical and functional development, neurological findings, and reproductive parameters. However, cesarean section data for the pregnant first generation (F 1) females in the high-dose group included significantly higher pre-implantation loss and significantly lower mean values for corpora lutea counts, number of implantations, and number of live embryos. Also, small testes and/or epididymides were observed in 1/20 and 2/20 first generation males in the mid- and high-dose groups respectively, and two of the affected animals failed to mate or induce pregnancy. However, the mean values for mating performance, fertility index, testes weight, testes and epididymides sperm counts, and epididymal sperm motility and progression were not altered in any of the F 1 males in benznidazole treatment groups. The number of live second generation (F 2) fetuses born to F 1 dams was reduced in the high-dose group. The benznidazole dose that was not associated with adverse effects was considered to be 50 mg/kg/day which is approximately equal to the MRHD based on whole body surface area comparison.
8.2 Lactation
Risk Summary
Limited published literature based on breast milk sampling reports that benznidazole is present in human milk at infant doses of 5.5 to 17% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.3-2.79. There are no reports of adverse effects on the breastfed infant and no information on the effects of benznidazole on milk production. Because of the potential for serious adverse reactions, and transmission of Chagas disease, advise patients that breastfeeding is not recommended during treatment with Benznidazole Tablets.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Benznidazole Tablets can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)] . Advise females of reproductive potential to use effective contraception during treatment with Benznidazole Tablets and for 5 days after the final dose.
Infertility
Males
Based on findings in rats, Benznidazole Tablets may impair fertility in males of reproductive potential. In rats, the effects on fertility were reversible 22 weeks after dosing; however, the effects in humans are unknown [see Nonclinical Toxicology ( 13.1)] .
8.4 Pediatric Use
The safety and effectiveness of Benznidazole Tablets have been established in pediatric patients 2 to 12 years of age for the treatment of Chagas disease. Use in pediatric patients 2 to 12 years of age was established in two adequate and well-controlled trials in pediatric patients 6 to 12 years old with additional safety and pharmacokinetic data from pediatric patients 2 to 6 years of age [see Dosage and Administration ( 2.2), Adverse Reactions ( 6.1), Clinical Studies ( 14)] .
Safety and effectiveness in pediatric patients below the age of 2 years and above the age of 12 years have not been established.
-
11 DESCRIPTION
Benznidazole Tablets contain benznidazole, a nitroimidazole antimicrobial. The chemical name of benznidazole is N-benzyl-2-(2-nitro-1H-imidazol-1-yl) acetamide. The empirical formula is C 12H 12N 4O 3 and the molecular weight is 260.246 g/mol. The structural formula is:
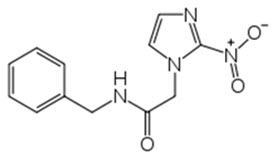
Figure 1: Benznidazole Structure
Benznidazole is a yellowish, practically crystalline powder that is practically insoluble in water, sparingly soluble in acetone and ethanol, and slightly soluble in methanol.
Benznidazole Tablets are white round tablets each containing 12.5 mg or 100 mg of benznidazole, for oral use. The 100 mg white tablets are round and functionally scored twice as a cross on both sides debossed with “E” on one side of each quarter portion. The 12.5 mg white tablets are round and unscored debossed with “E” on one side.
The inactive ingredients are as follows: magnesium stearate, NF; microcrystalline cellulose, NF; monohydrate lactose, NF; pre-gelatinized corn starch, NF; and sodium croscarmellose, NF.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Benznidazole is a nitroimidazole antimicrobial drug [see Microbiology ( 12.4)] .
12.3 Pharmacokinetics
Absorption
The absorption of benznidazole from three different 100 mg benznidazole preparations was comparable when administered as a single dose under fasting conditions in adult healthy volunteers ( Table 6).
Table 6: Summary of Benznidazole Pharmacokinetic (PK) Parameters Following a Single Oral Dose of 100 mg Benznidazole Administered Under Fasting Conditions in Adult Healthy Volunteers. Preparations for 100 mg Benznidazole Oral Dose One Benznidazole 100 mg Tablet Taken Whole Slurry prepared with one Benznidazole 100 mg Tablet Slurry prepared with eight Benznidazole 12.5 mg Tablets Parameter Mean (SD) Mean (SD) Mean (SD) Cmax (mg/L) 2.4 (0.5) 2.4 (0.4) 2.4 (0.4) Tmax a(h) 2 (1-4) 2 (0.5-4) 2 (1 - 4.5) AUC (mg*h/L) 43.5 (9.0) 41.8 (9.6) 44.1 (11.8) a Tmax is presented as median (range)
Effect of Food
Benznidazole Cmax and AUC were not affected by the administration of Benznidazole 100 mg tablet with a high-fat, high-caloric meal (approximately 1034 total kcal, 67 kcal from fat, 42 kcal from carbohydrates, 59 kcal from protein) compared with fasted conditions in adult healthy volunteers. Serum concentrations of benznidazole reached peak levels at 3.2 hours (1-10 hours) after administration of Benznidazole Tablets 100 mg tablet after a high-fat, high-caloric meal, and at 2.0 hours (0.5-4 hours) in fasted conditions [see Dosage and Administration ( 2.1)] .
Distribution
Protein binding is reported to be approximately 44 to 60 %. The mean blood:plasma radioactivity ratios, ranging from 0.63 to 1.10, suggest that there is no preferential distribution of benznidazole into the red blood cells. The apparent volume of distribution of benznidazole is approximately 45 L.
Elimination
The elimination half-life of benznidazole is approximately 13 hours in healthy volunteers following single dose. The apparent total clearance of benznidazole is about 2.3 L/h.
Metabolism
In plasma, benznidazole parent drug is the most abundant component, accounting for approximately 80% of the total drug related exposure and no major circulating metabolites were identified. The primary benznidazole metabolism pathway is indicated to be nitro reduction followed by glucuronidation.Excretion
Following oral administration of 100 mg radiolabeled benznidazole, about 68% and 21% of the radioactivity is recovered in urine (6% of total dose as unchanged parent drug) and feces (unchanged parent drug not detected), respectively.
Specific Populations
The effect of sex, race, renal impairment, or hepatic impairment on the pharmacokinetics of benznidazole is unknown.
Drug Interaction Studies
In vitro studies showed that benznidazole is a P-gp substrate and does not notably induce Cytochrome P450 enzymes 1A2, 2B6, and 3A4 at concentrations up to 100 mcM.
12.4 Microbiology
Mechanism of Action
Benznidazole inhibits the synthesis of DNA, RNA, and proteins within the T. cruzi parasite. Studies suggest that benznidazole is reduced by a Type I nitroreductase (NTR) enzyme of T. cruzi producing a series of short-lived intermediates that may promote damage to several macromolecules including DNA. In mammalian cells, however, benznidazole is metabolized by reduction of the nitro group to an amino group by a Type II NTR enzyme. The precise mechanism of action is not known.
Antimicrobial Activity
Benznidazole is active against all three stages, trypomastigotes, amastigotes, and epimastigotes, of T. cruzi. However, the sensitivity of T. cruzi strains to benznidazole, from different geographic regions, may vary.
Resistance
Studies in vitro and in mice infected with T. cruzi suggest a potential for development of resistance to benznidazole.
The mechanisms of drug resistance appear to be multifactorial. These mechanisms include decreased activity due to a mutation in the nitroreductase ( TcNTR) gene. Other mechanisms include higher efflux activity due to over expression of TcPGP 1 and TcPGP 2 genes that encode p-glycoprotein as well as TcABCG1 genes that encode ATP-binding cassette transporters. Also, some studies reported overexpression of other genes TcFeSOD-A and TcCyP19 that encode superoxide dismutase and cyclophilin, respectively, which have diverse biological function and may help parasite survival. However, the clinical relevance of these findings is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term carcinogenicity studies for benznidazole have not been performed.
Nitroimidazoles, which have similar chemical structures to benznidazole have been reported to be carcinogenic in mice and rats.
Genetic Toxicity
Genotoxicity of benznidazole has been demonstrated in vitro in several bacterial species and mammalian cell systems and in vivo in mammals.
Benznidazole was mutagenic in several strains of S. typhimurium (TA 100, 102 1535, 1537, 1538, 97, 98 99 53 and UTH8414), E.coli, and K. pneumoniae.
Benznidazole was genotoxic in several in vitro mammalian cell assays including a chromosome aberration assay in human lymphocytes and in sister chromatid exchange assays in human lymphocytes and in Human Hep G2 cells.
In vivo, benznidazole was shown to be positive for genotoxicity in a mouse bone marrow micronucleus assay, in mouse and human red blood cell micronucleus assays, in a mouse abnormal sperm head assay and in a human peripheral blood lymphocyte assay. However in other micronucleus studies in mice and rats, oral doses of benznidazole did not cause a significant increase in the frequency of chromosomal aberrations in bone marrow cells or micronuclei in peripheral blood cells.
Impairment of Fertility
In a 6-month, chronic repeated-dosing study with Wistar rats, benznidazole was shown to produce dose-dependent testicular and epididymal atrophy at a dose of 30 mg/kg/day (approximately equivalent to 0.6 times the MRHD based on whole body surface area comparisons). Aspermia was also evident in affected rats, but fertility was not assessed in this study. The benznidazole dose that was not associated with adverse effects in this study was considered to be 10 mg/kg/day (5 mg/kg twice daily) in males which is approximately 0.2-times the MRHD based on whole body surface area comparison. In other literature reports, benznidazole has been shown to cause testicular atrophy and inhibit spermatogenesis in pubertal and adult rats and mice 5-7.
In a male fertility study, benznidazole was administered by oral (gavage) administration in daily doses of 10, 30, and 100 mg/kg/day to male Wistar rats beginning 10 weeks before mating, through mating, and for two weeks after mating for a total of 14-weeks. At the end of the dosing period, testicular and epididymal weights and the percent of normal sperm were decreased in mid- and high-dose males compared to control values. Also altered gonadal function, mating behavior and reduced fertility associated with morphologically and functionally altered sperm and histological changes in the testes and epididymides was observed in high-dose males. However, after a 22-week treatment-free period, almost complete recovery was observed with fully restored fertility compared to control values. After the 22-week treatment-free period the remaining benznidazole-related effects included reduced testicular and epididymal weights (10-22% reduction in absolute weight) compared to control values at the high benznidazole dose of 100 mg/kg/day (equivalent to approximately 2 times the MRHD based on whole body surface area comparison). The benznidazole dose at which no adverse effect was observed was 10 mg/kg/day which is equivalent to approximately 0.2 times the MRHD based on whole body surface area comparison.
In a female fertility study, benznidazole was administered by oral gavage to female Wistar rats in doses of 15, 50, and 150 mg/kg/day for a 2-week pre-mating period, during mating, and through day 7 of gestation. The high dose was associated with transient lower body weight gain and food consumption. There were no benznidazole-related effects on mating performance or fertility and no adverse macroscopic or reproductive organ weight changes. However, higher post-implantation loss with lower live litter size was observed at the high dose of 150 mg/kg/day (equivalent to approximately 3 times the MRHD based on whole body surface area comparison). The benznidazole dose that was not associated with adverse effects for this study was considered to be 50 mg/kg/day which is approximately equivalent to the MRHD based on whole body surface area comparison.13.2 Animal Toxicology and/or Pharmacology
Single oral dose toxicity studies in rats have established that benznidazole causes ultrastructural changes in the adrenal cortex, colon, esophagus, ovaries, and testis 5, 8-11. In these tissues, these changes were associated with the presences of nitro reductase activity, the production of reactive metabolites, and/or covalent binding of metabolites.
Neurotoxicity including brain axonal degeneration and Purkinje cell degeneration was observed with repeated-oral dosing in dogs without adverse changes in peripheral nerves 12-14. Neurological signs included: apathy, hypertonia, hyperreflexia, ataxia, loss of balance, oscillatory movements of the trunk and head, strong contractions of the back and leg muscles, opisthotonos and nystagmus. Neurotoxicity was not observed in other test species, including mouse, rat, guinea pig, and rabbit.
-
14 CLINICAL STUDIES
The safety and effectiveness of benznidazole for the treatment of Chagas disease in patients 6 to 12 years of age was established in two adequate and well-controlled trials (Trial 1 and Trial 2) as described below.
Trial 1 was a randomized, double-blind, placebo-controlled trial in children 6 to 12 years of age with chronic indeterminate Chagas disease conducted in Argentina. The chronic indeterminate form of Chagas disease includes patients with serologic evidence of T. cruzi infection without symptoms of cardiac or gastrointestinal disease. A total of 106 patients were randomized to receive either benznidazole (5 mg/kg/day for 60 days) or placebo and followed for 4 years. Patients with at least two positive conventional serologic tests for antibodies to T. cruzi were included in the study. The conventional serologic tests used include indirect hemagglutination assay (IHA), immunofluorescence antibody assay (IFA), and/or enzyme linked immunosorbent assay (ELISA) and were based on the detection of antibodies against T. cruzi parasites.
Trial 2 was a randomized, double-blind, placebo-controlled trial in pediatric patients 7 to 12 years of age with chronic indeterminate Chagas disease conducted in Brazil. A total of 129 patients were randomized to receive either benznidazole (7.5 mg/kg/day for 60 days) or placebo and followed for 3 years. Patients with three positive conventional serologic tests for antibodies to T. cruzi were included in the study. The conventional serologic tests include IHA, IFA, and/or ELISA and were based on the detection of antibodies against T. cruzi parasites.
Both trials measured antibodies by conventional and nonconventional assays. The nonconventional assays include F29-ELISA and AT- chemiluminescence-ELISA that are based on detection of anti- T. cruzi IgG antibodies against the recombinant antigens, F29 and AT from the flagella of T. cruzi parasites. Benznidazole treatment resulted in a significantly higher percentage of seronegative patients by a nonconventional assay. Results at the end of follow-up are reported in the following table.
Table 7: Nonconventional ELISA a Serologic Status at End-of-Follow-Up (mITT population b) a Enzyme-linked immunosorbent assay (F29 ELISA in Study Trial 1 and AT chemiluminescence-ELISA in Trial 2); the F29 and AT antigens represent antigens from the flagella of T. cruzi parasites.
b Modified intent to treat (mITT) population includes subjects who are positive for the assay at baseline;
c Exact confidence intervals presented.Benznidazole
Placebo
Difference (95% CI)c
Trial 1
N=40
N=37
Seronegative
24 (60.0)
5 (13.5)
46.5 (24.5, 64.4)
Seropositive
15
29
Missing
1
3
Trial 2
N=64
N=65
Seronegative
35 (54.7)
3 (4.6)
50.1 (35.8, 63.4)
Seropositive
23
51
Missing
6
11
In Trial 1 using conventional ELISA, 4 of 53 (7.5%) benznidazole subjects and 2 of 50 (4.0%) placebo subjects seroconverted to negative by the end of follow-up (difference 3.5, 95% CI (-7.0, 14.9)). In Trial 2 using conventional ELISA, 4 of 64 (6.3%) of benznidazole subjects and 0 of 65 placebo subjects seroconverted to negative by the end of follow-up (difference 6.3, 95% CI (0.3, 15.2)).
-
15 REFERENCES
- Sosa Estani S, et al., 1998, Efficacy of Chemotherapy with Benznidazole in Children in the Indeterminate Phase of Chagas' Disease. Am J Trop Med Hyg 59: 526-529.
- de Andrade, Ana Lucia S. Sgambatti, et al., 1996, Randomised Trial of Efficacy of Benznidazole in Treatment of Early Trypanosoma cruzi Infection. Lancet 348: 1407-1413.
- Altcheh, Jaime, et al., 2014, Population Pharmacokinetic Study of Benznidazole in Pediatric Chagas Disease Suggests Efficacy Despite Lower Plasma Concentrations than in Adults. PLoS Negl Trop Dis 8:e2907.
- García-Bournissen, F, S Moroni, ME Marson, et al., 2015, Limited Infant Exposure to Benznidazole Through Breast Milk During Maternal Treatment for Chagas Disease. Arch Dis Child 100:90-94.
- Bernacchi, AS, CR de Castro, EG de Toranzo, and JA Castro, 1986, Effects of Nifurtimox or Benznidazole Administration on Rat Testes: Ultrastructural Observations and Biochemical Studies, Exp Mol Pathol 45: 245-256.
- Vieira, CL, TL Lamano-Carvalho, AL Favaretto, MM Valenca, J Antunes-Rodridgues, and AA Barreira, 1989, Testes Alterations in Pubertal Benznidazole-treated Rats, Braz J Med Biol Res, 22: 695-698.
- Navarro, ML and R Nagel, 1990, Abnormal Sperm Induced in Mice by Oral Administration of Antichagasic Drugs, Comunicaciones Biologicas, 8: 251-258.
- de Castro, RC, EG Diaz de Toranzo, and JA Castro, 1992, Benznidazole-induced Ultrastructural Alterations in Rat Adrenal Cortex: Mechanistic Studies, Toxicology, 74: 223-232.
- Diaz, EG, RC de Castro, M Montalto de Mecca, and JA Castro, 2000, Benznidazole-induced Ultrastructural and Biochemical Alterations in Rat Colon, Acta Pharmacol Sin, 21: 961-966.
- de Castro, RC, M Montalto de Mecca, SL Fanelli, EC de Ferreyra, EG Diaz, and JA Castro, 2003, Benznidazole-induced Ultrastructural and Biochemical Alterations in Rat Esophagus, Toxicology, 191: 189-198.
- de Castro, CR, EGD de Toranzo, AS Bernacchi, M Carbone, and JA Castro, 1989, Ultrastructural Alterations in Ovaries from Nifurtimox or Benznidazole-treated Rats: Their Relation to Ovarian Nitroreductive Biotransformation of Both Drugs, Exp Mol Pathol, 50: 385-397.
- Scharer, K, 1972, Selective Purkinje Cell Damage in Dogs after Oral Administration of High Doses of Nitroimidazole Derivatives, Verhandlungen der Deutschen Gesellschaft fur Pathologie, 56: 407-10.
- Flores-Vieira, CLL and AA Barreira, 1997a, Experimental Benznidazole Encephalopathy: I. Clinical-Neurological Alterations, J Neurol Sci, 150: 3-11.
- Flores-Vieira, CLL, L Chimelli, RMF Fernandez, and AA Barreira, 1997b, Experimental Benznidazole Encephalopathy: II. Electroencephalographic and Morphological Alterations, J Neurol Sci, 150:13-25.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Benznidazole Tablets (12.5 mg or 100 mg) are supplied as follows:
- 100 mg white tablets, round and functionally scored twice as a cross on both sides. Each tablet is about 10 mm in diameter debossed with “E” on one side of each quarter portion.
- 12.5 mg white tablets, round and unscored. Each tablet is about 5 mm in diameter debossed with “E” on one side.
Benznidazole Tablets 100 mg are available in bottles of 100 tablets (NDC 0642-7464-10).
Benznidazole Tablets 12.5 mg are available in bottles of 100 tablets (NDC 0642-7463-12).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Instructions for Use).
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure to Benznidazole Tablets during pregnancy can result in fetal harm.
Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions ( 5.2) and Use in Specific Populations ( 8.1)] .
Advise females of reproductive potential to use effective contraception while taking
Benznidazole Tablets and for 5 days after the last dose [see Use in Specific Populations ( 8.3)] .
Lactation
Advise women not to breastfeed during treatment with Benznidazole Tablets [see Use in Specific Populations ( 8.2)] .
Infertility
Advise males of reproductive potential that Benznidazole Tablets may impair fertility [see Use in Specific Populations ( 8.3) and Nonclinical Toxicology ( 13.1)] .
Important Administration Instructions
Advise patients and parents/caregivers of pediatric patients taking Benznidazole Tablets that:
•Benznidazole Tablets 100 mg are functionally scored tablets which can be split into one-half (50 mg) or one-quarter (25 mg) at the scored lines to provide doses less than 100 mg.
•Benznidazole Tablets 12.5 mg and 100 mg (whole or split) can be made into a slurry in a specified volume of water for the pediatric population [see Dosage and Administration ( 2.3)] .
Hypersensitivity Skin Reactions
Advise patients that serious skin reactions can occur with Benznidazole Tablets. In case of skin reactions, presenting with additional symptoms of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is necessary.
Central and Peripheral Nervous System Effects
Advise patients that treatment can potentially cause paresthesia or symptoms of peripheral neuropathy. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended.
Hematological Manifestations of Bone Marrow Depression
Advise patients that there have been hematological manifestations of bone marrow depression, such as anemia and leukopenia, which are reversible, and normalized after treatment discontinuation.
Manufactured for Chemo Research, S.L.
Madrid, SpainManufactured by Laboratorios Liconsa S.A.
Guadalajara, SpainDistributed by:

Exeltis USA, Inc.
Florham Park, NJ 07932 -
INSTRUCTIONS FOR USE
BENZNIDAZOLE
tablets, for oral useRead this Instructions for Use before you start taking BENZNIDAZOLE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Note:
• Your doctor may need to change your dose of BENZNIDAZOLE during treatment as needed.
• BENZNIDAZOLE 100 mg tablets can be taken whole or broken at scored lines.
• BENZNIDAZOLE 100 mg tablets are marked with scored lines and may be broken at these scored lines to provide the following doses: 75 mg, 50 mg and 25 mg.
100 mg treatment (take the whole tablet)
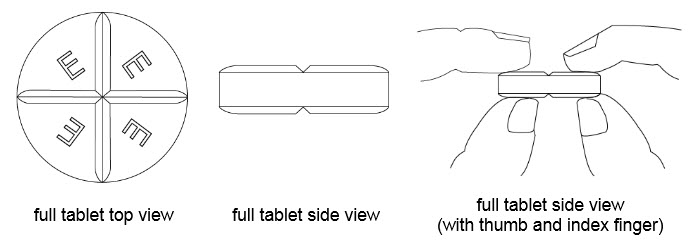
How to break your BENZNIDAZOLE 100 mg tablet:
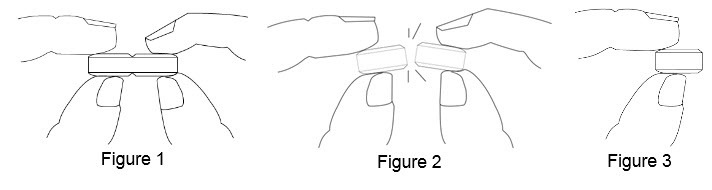
• Hold the tablet between your thumbs and index fingers close to the scored line ( See Figure 1).
• Apply enough pressure to break the tablet at the scored line ( See Figure 2).
• Only use a tablet that has been broken at the scored line ( See Figure 3).
• Do not break the BENZNIDAZOLE 100 mg tablet in any other way.
75 mg treatment (take one-half of the tablet and one-fourth of the tablet)
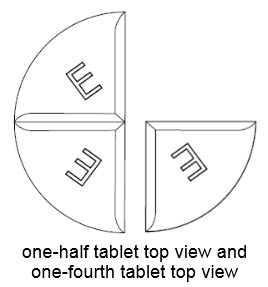
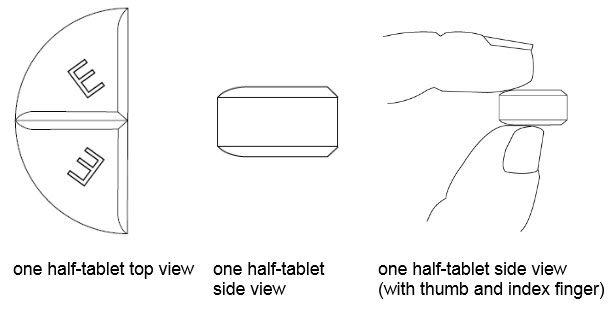
50 mg treatment (take one-half of the tablet)
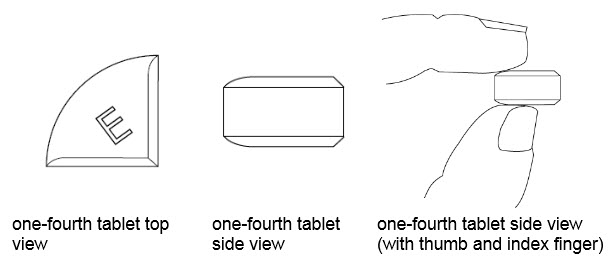
25 mg treatment (take one-fourth of the tablet)
How should I store BENZNIDAZOLE?
• Store BENZNIDAZOLE at room temperature 20° to 25°C (68°to 77°F).
• Keep BENZNIDAZOLE in the bottle that it comes in and keep the bottle tightly closed.
• Keep BENZNIDAZOLE away from moisture.
Keep BENZNIDAZOLE and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Laboratori os Liconsa S.A., Guadalajara, Spain
Issued: August 2017
-
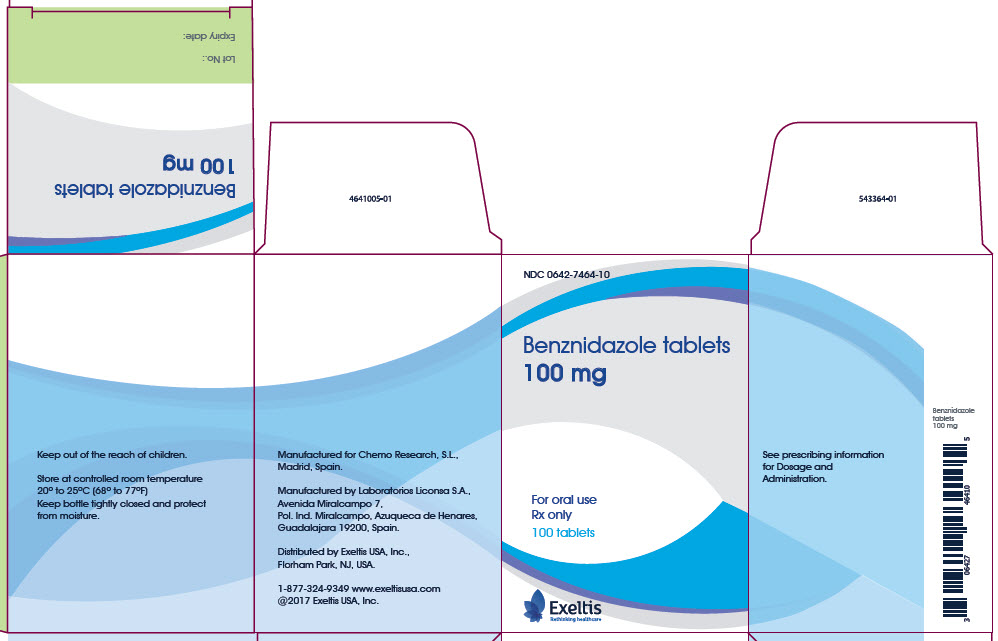
PRINCIPAL DISPLAY PANEL - 100 mg CARTON
NDC 0642-7464-10
Benznidazole tablets
100 mgFor oral use
Rx only
100 tabletsExeltis
Rethinking healthcareKeep out of the reach of children.
Store at controlled room temperature
20° to 25°C (68° to 77°F)
Keep bottle tightly closed and protect
from moisture.Manufactured for Chemo Research, S.L.,
Madrid, Spain.Manufactured by Laboratorios Liconsa S.A.,
Avenida Miralcampo 7,
Pol. Ind. Miralcampo, Azuqueca de Henares,
Guadalajara 19200, Spain.Distributed by Exeltis USA, Inc.,
Florham Park, NJ, USA.1-877-324-9349 www.exeltisusa.com
©2017 Exeltis USA, Inc.See prescribing information
for Dosage and
Administration.Benznidazole
tablets
100 mgBenznidazole tablets
100 mgLot No.:
Expiry date: -
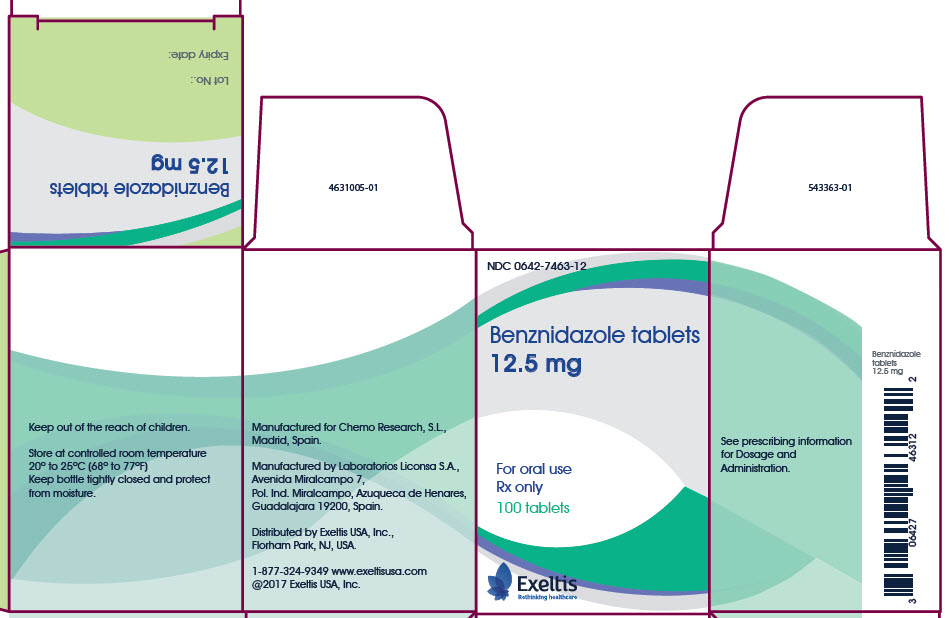
PRINCIPAL DISPLAY PANEL - 12.5 mg CARTON
NDC 0642-7463-12
Benznidazole tablets
12.5 mgFor oral use
Rx only
100 tabletsExeltis
Rethinking healthcareKeep out of the reach of children.
Store at controlled room temperature
20° to 25°C (68° to 77°F)
Keep bottle tightly closed and protect
from moisture.Manufactured for Chemo Research, S.L.,
Madrid, Spain.Manufactured by Laboratorios Liconsa S.A.,
Avenida Miralcampo 7,
Pol. Ind. Miralcampo, Azuqueca de Henares,
Guadalajara 19200, Spain.Distributed by Exeltis USA, Inc.,
Florham Park, NJ, USA.1-877-324-9349 www.exeltisusa.com
©2017 Exeltis USA, Inc.See prescribing information
for Dosage and
Administration.Benznidazole
tablets
12.5 mgBenznidazole tablets
12.5 mgLot No.:
Expiry date: -
INGREDIENTS AND APPEARANCE
BENZNIDAZOLE
benznidazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0642-7464 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZNIDAZOLE (UNII: YC42NRJ1ZD) (BENZNIDAZOLE - UNII:YC42NRJ1ZD) BENZNIDAZOLE 100 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color white Score 4 pieces Shape ROUND Size 10mm Flavor Imprint Code E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0642-7464-10 1 in 1 CARTON 03/30/2018 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209570 03/30/2018 BENZNIDAZOLE
benznidazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0642-7463 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZNIDAZOLE (UNII: YC42NRJ1ZD) (BENZNIDAZOLE - UNII:YC42NRJ1ZD) BENZNIDAZOLE 12.5 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color white Score no score Shape ROUND Size 5mm Flavor Imprint Code E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0642-7463-12 1 in 1 CARTON 03/30/2018 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209570 03/30/2018 Labeler - Exeltis USA, Inc. (071170534) Registrant - Exeltis USA, Inc. (071170534)