Label: METHOCARBAMOL AND ASPIRIN tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50564-490-01, 50564-490-05 - Packager: Jerome Stevens Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each tablet, for oral administration contains:
Methocarbamol, USP 400 mg
Aspirin, USP 325 mgInactive Ingredients: Pregelatinized starch, povidone, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, stearic acid.
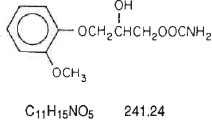
The chemical name of methocarbamol is 3-(2-meth-oxyphenoxy)-1,2-propanediol 1-carbamate and has the chemical formula C11H15NO5. Its molecular weight is 241.24. Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane. The structural formula is shown below.
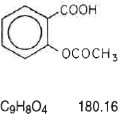
The aspirin component is 2-(acetyloxy)-, Benzoic acid, a white crystal, commonly tabular or needle-like, or white, crystalline powder. Is odorless or has a faint odor. Is stable in dry air; in moist air it gradually hydrolyzes to salicylic and acetic acids. Slightly soluble in water; freely soluble in alcohol; soluble in chloroform and in ether; sparingly soluble in absolute ether and is represented by the following structural formula:
-
CLINICAL PHARMACOLOGY
Methocarbamol and Aspirin provides a double approach to the management of discomforts associated with musculoskeletal disorders.
Methocarbamol
The mechanism of action of methocarbamol in humans has not been established, but may be due to general central nervous system depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
Aspirin
Aspirin (acetylsalicylic acid) works by inhibiting the body's production of prostaglandins, including prostaglandins involved in inflammation. Prostaglandins cause pain sensations by stimulating muscle contractions and dilating blood vessels throughout the body. In the CNS, aspirin works on the hypothalamus heat-regulating center to reduce fever, however, other mechanisms may be involved.
-
PHARMACOKINETICS
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg, the mean plasma elimination half-life ranges between 1 and 2 hours, and the plasma protein binding ranges between 46% and 50%.
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine. Small amounts of unchanged methocarbamol also are excreted in the urine.
Aspirin is hydrolyzed primarily to salicylic acid in the gut wall and during first-pass metabolism through the liver. Salicylic acid is absorbed rapidly from the stomach, but most of the absorption occurs in the proximal small intestine. Following absorption, salicylate is distributed to most body tissues and fluids, including fetal tissues, breast milk, and the CNS. High concentrations are found in the liver and kidneys. Salicylate is variably bound to serum proteins, particularly albumin.
The biotransformation of aspirin occurs primarily in the liver by the microsomal enzyme system. With a plasma half-life of approximately 15 minutes, aspirin is rapidly hydrolyzed to salicylate. At low doses, salicylate elimination follows first-order kinetics. The plasma half-life of salicylate is approximately 2 to 3 hours.
Approximately 10% of aspirin is excreted as unchanged salicylate in the urine. The major metabolites excreted in the urine are salicyluric acid (75%), salicyl phenolic glucuronide (10%), salicyl acyl glucuronide (5%), and gentisic and gentisuric acid (less than 1%) each. Eighty to 100% of a single dose is excreted in the urine within 24 to 72 hours.
-
SPECIAL POPULATIONS
Elderly
The mean (± SD) elimination half-life of methocarbamol in elderly healthy volunteers (mean (± SD) age, 69 (± 4) years) was slightly prolonged compared to a younger (mean (± SD) age, 53.3 (± 8.8) years), healthy population (1.5 (± 0.4) hours versus 1.1 (± 0.27) hours, respectively). The fraction of bound methocarbamol was slightly decreased in the elderly versus younger volunteers (41 to 43% versus 46 to 50%, respectively).
Renally Impaired
The clearance of methocarbamol in 8 renally-impaired patients on maintenance hemodialysis was reduced about 40% compared to 17 normal subjects, although the mean (± SD) elimination half-life in these two groups was similar: 1.2 (± 0.6) versus 1.1 (± 0.3) hours, respectively.
Avoid aspirin in patients with severe renal impairment (glomerular filtration rate less than 10 mL/minute).
Hepatically Impaired
In 8 patients with cirrhosis secondary to alcohol abuse, the mean total clearance of methocarbamol was reduced approximately 70% compared to that obtained in 8 age- and weight-matched normal subjects. The mean (± SD) elimination half-life in the cirrhotic patients and the normal subjects was 3.38 (± 1.62) hours and 1.11 (± 0.27) hours, respectively. The percent of methocarbamol bound to plasma proteins was decreased to approximately 40 to 45% compared to 46 to 50% in the normal subjects.
Avoid aspirin in patients with severe hepatic impairment.
-
INDICATIONS AND USAGE
Methocarbamol and Aspirin tablets are indicated as an adjunct to rest, physical therapy and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
- CONTRAINDICATIONS
-
WARNINGS
Methocarbamol
Since methocarbamol may possess a general central nervous system depressant effect, patients receiving methocarbamol should be cautioned about combined effects with alcohol and other CNS depressants.
Aspirin
Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs, including Methocarbamol and Aspirin Tablets , in pregnant women at about 30 weeks gestation and later. NSAIDs including Methocarbamol and Aspirin Tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment:
Use of NSAIDs, including Methocarbamol and Aspirin Tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation.
Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some post marketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit Methocarbamol and Aspirin Tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if Methocarbamol and Aspirin Tablets treatment extends beyond 48 hours. Discontinue Methocarbamol and Aspirin Tablets if oligohydramnios occurs and follow up according to clinical practice (see PRECAUTIONS; Pregnancy).
Use In Activities Requiring Mental Alertness
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as Methocarbamol and Aspirin Tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively presents with fever, rash, lymphadenopathy and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue Methocarbamol and Aspirin Tablets and evaluate the patient immediately.
-
PRECAUTIONS
General
Products containing aspirin should be administered with caution to patients with gastritis or peptic ulceration, or those receiving hypoprothrombinemic anticoagulants.
Aspirin has been associated with elevated hepatic enzymes, blood urea nitrogen and serum creatinine, hyperkalemia, proteinuria, and prolonged bleeding time.
Hemorrhage
Aspirin may increase the likelihood of hemorrhage due to its effect on the gastric mucosa and platelet function (prolongation of bleeding time). Salicylates should be used with caution in the presence of peptic ulcer or coagulation abnormalities.
Information for Patients
Methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
Fetal Toxicity
Inform pregnant women to avoid use of aspirin and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with Methocarbamol and Aspirin Tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours (see WARNINGS, Fetal Toxicity, PRECAUTIONS; Pregnancy).
Serious Skin Reactions, including DRESS
Advise patients to stop taking Methocarbamol and Aspirin Tablets immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible (see WARNINGS).
Drug Interactions
See WARNINGS and PRECAUTIONS for interaction with CNS drugs and alcohol.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Angiotensin Converting Enzyme (ACE) Inhibitors: The hyponatremic and hypotensive effects of ACE inhibitors may be diminished by the concomitant administration of aspirin due to its indirect effect on the renin-angiotensin conversion pathway.
Acetazolamide: Concurrent use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide (and toxicity) due to competition at the renal tubule for secretion.
Anticoagulant Therapy (Heparin and Warfarin): Patients on anticoagulation therapy are at increased risk for bleeding because of drug-drug interactions and the effect on platelets. Aspirin can displace warfarin from protein binding sites, leading to prolongation of both the prothrombin time and the bleeding time. Aspirin can increase the anticoagulant activity of heparin, increasing bleeding risk.
Anticonvulsants: Salicylate can displace protein-bound phenytoin and valproic acid, leading to a decrease in the total concentration of phenytoin and an increase in serum valproic acid levels.
Beta Blockers: The hypotensive effects of beta blockers may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow, and salt and fluid retention.
Diuretics: The effectiveness of diuretics in patients with underlying renal or cardiovascular disease may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention.
Methotrexate: Aspirin may enhance the serious side and toxicity of methotrexate due to displacement from its plasma protein binding sites and/or reduced renal clearance.
Nonsteroidal Anti-inflammatory Drugs (NSAID's): The concurrent use of aspirin with other NSAID's should be avoided because this may increase bleeding or lead to decreased renal function. Aspirin may enhance the serious side effects and toxicity of ketorolac, due to displacement from its plasma protein binding sites and/or reduced renal clearance.
Oral Hypoglycemics Agents: Aspirin may increase the serum glucose-lowering action of insulin and sulfonylureas leading to hypoglycemia.
Uricosuric Agents: Salicylates antagonize the uricosuric action of probenecid or sulfinpyrazone.
Drug/Laboratory Test Interactions
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Salicylates may increase the protein bound iodine (PBI) result by competing for the protein binding sites on pre-albumin and possibly thyroid-binding globulins.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Long-term studies in animals to evaluate the carcinogenic potential of aspirin have not been conducted. Aspirin induced chromosome aberrations in cultured human fibroblasts. Aspirin has been shown to inhibit ovulation in rats.
Pregnancy
Teratogenic effects – Pregnancy Category C
Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol should be given to a pregnant woman only if clearly needed.
Safe use of Methocarbamol has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, Methocarbamol should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see WARNINGS).
Nonteratogenic Effects
Risk Summary
Use of NSAIDs, including aspirin, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of Methocarbamol and Aspirin Tablets use between 20 and 30 weeks of gestation, and avoid Methocarbamol and Aspirin Tablets use at about 30 weeks of gestation and later in pregnancy [see WARNINGS, Fetal Toxicity].
-
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including aspirin, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive.
Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as aspirin, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal/ Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including Methocarbamol and Aspirin Tablets, can cause premature closure of the fetal ductus arteriosus (see WARNINGS, Fetal Toxicity).
Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If Methocarbamol and Aspirin Tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue Methocarbamol and Aspirin Tablets and follow up according to clinical practice (see WARNINGS, Fetal Toxicity).
Data
Human Data
Premature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal/ Renal Impairment:
Published studies and post marketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these post marketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Labor or Delivery
Salicylates readily cross the placenta and by inhibiting prostaglandin synthesis, may cause constriction of ductus arteriosus resulting in pulmonary hypertension and increased fetal mortality and, possibly other untoward fetal effects. Aspirin use in pregnancy can also result in alteration in maternal and neonatal hemostasis mechanisms. Maternal aspirin use during later stages of pregnancy may cause low birth weight, increased incidence of intracranial hemorrhage in premature infants, stillbirths and neonatal death. Use during pregnancy, especially in the third trimester, should be avoided.
Nursing Mothers
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk.
Salicylic acid has been detected in breast milk. Adverse effects on platelet function in the nursing infant exposed to aspiring in breast milk may be a potential risk. Furthermore, the risk of Reye Syndrome cause by salicylate in breast milk is unknown.
Because many drugs are excreted in human milk, caution should be exercised when Methocarbamol and Aspirin Tablets are administered to a nursing woman.
-
ADVERSE REACTIONS
Adverse reactions reported coincident with the administration of methocarbamol include:
Body as a whole
Anaphylactic reaction, angioneurotic edema, fever, headache
Cardiovascular system
Bradycardia, flushing, hypotension, syncope, thrombophlebitis
Digestive system
Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting
Hemic and lymphatic system
Leukopenia
Immune system
Hypersensitivity reactions
Nervous system
Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular
incoordination, nystagmus, sedation, seizures (including grand mal), vertigo
Skin and special senses
Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticaria
Aspirin
Aspirin may increase the likelihood of hemorrhage due to its effect on the gastric mucosa and platelet function. Furthermore, aspirin has the potential to cause anaphylaxis in hypersensitive patients as well as angioedema especially in patients with chronic urticaria. Other adverse reactions due to aspirin use include anorexia, reversible hepatotoxicity, leukopenia, thrombocytopenia, purpura, decreased plasma iron concentration, and shortened erythrocyte survival time.
Adverse reactions that have been associated with the use of aspirin include: nausea and other gastrointestinal discomfort, gastritis, gastric erosion, vomiting, constipation, diarrhea, angio-edema, asthma, rash, pruritis, urticaria. Gastrointestinal discomfort may be minimized by taking Methocarbamol and Aspirin with food.
To report SUSPECTED ADVERSE REACTIONS, contact Jerome Stevens Pharmaceuticals Inc. at 1-844-686-1019 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Limited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following symptoms: nausea, drowsiness, blurred vision, hypotension, seizures, and coma.
In post-marketing experience, deaths have been reported with an overdose of methocarbamol alone or in the presence of other CNS depressants, alcohol or psychotropic drugs.
Early signs of acute aspirin (salicylate) overdose including tinnitus occur at plasma concentrations approaching 200 mcg/mL. Plasma concentrations of aspirin above 300 mcg/mL are toxic. Severe toxic effects are associated with levels above 400 mcg/mL. A single lethal dose of aspirin in adults is not known with certainty but death may be expected at 30 g. For real or suspected overdose, a Poison Control Center should be contacted immediately.
In acute salicylate overdose, severe acid-base and electrolyte disturbances may occur and are complicated by hyperthermia and dehydration, and coma. Respiratory alkalosis occurs early while hyperventilation is present, but is quickly followed by metabolic acidosis. Serious symptoms such as depression, coma, and respiratory failure progress rapidly.
Salicylism (chronic salicylate toxicity) may be noted by symptoms such as dizziness, tinnitus, difficulty hearing, nausea, vomiting, diarrhea, and mental confusion. More severe salicylism may result in respiratory alkalosis.
Treatment of Overdosage: Supportive therapy for 24 hours, as methocarbamol is excreted within that time. If salicylate intoxication occurs, especially in children, the hyperpnea may be controlled with sodium bicarbonate. Judicious use of 5% CO2 with 95% O2 may be of benefit. Abnormal electrolyte patterns should be corrected with appropriate fluid therapy.
-
DOSAGE AND ADMINISTRATION
Adults and children over 12 years of age: Two tablets four times daily. Three tablets four times daily may be used in severe conditions for one to three days in patient who are able to tolerate salicylates. These dosage recommendations provide respectively 3.2 and 4.8 grams of methocarbamol per day.
-
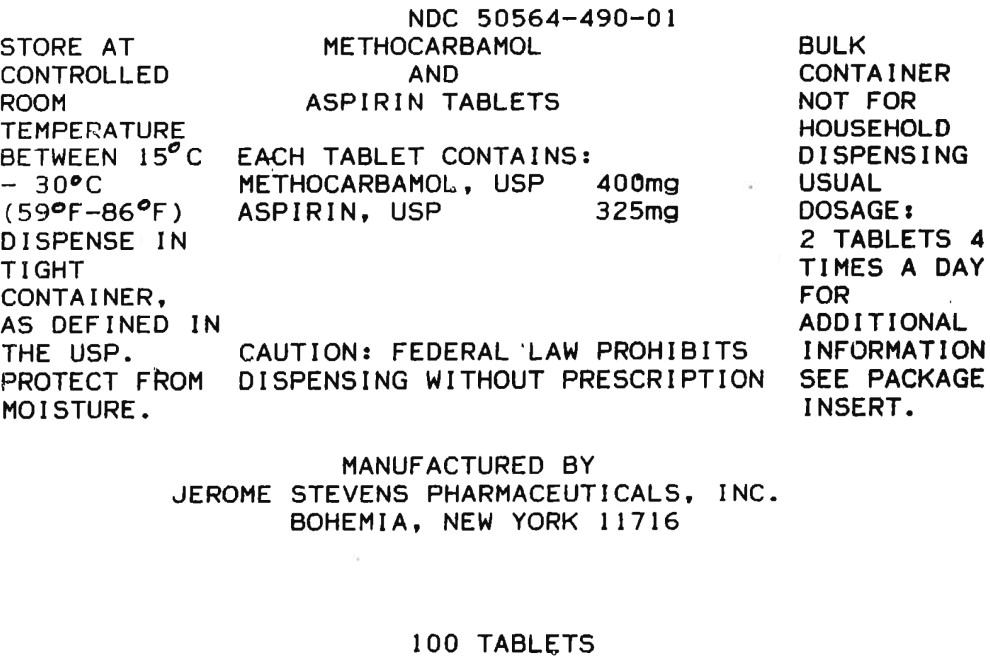
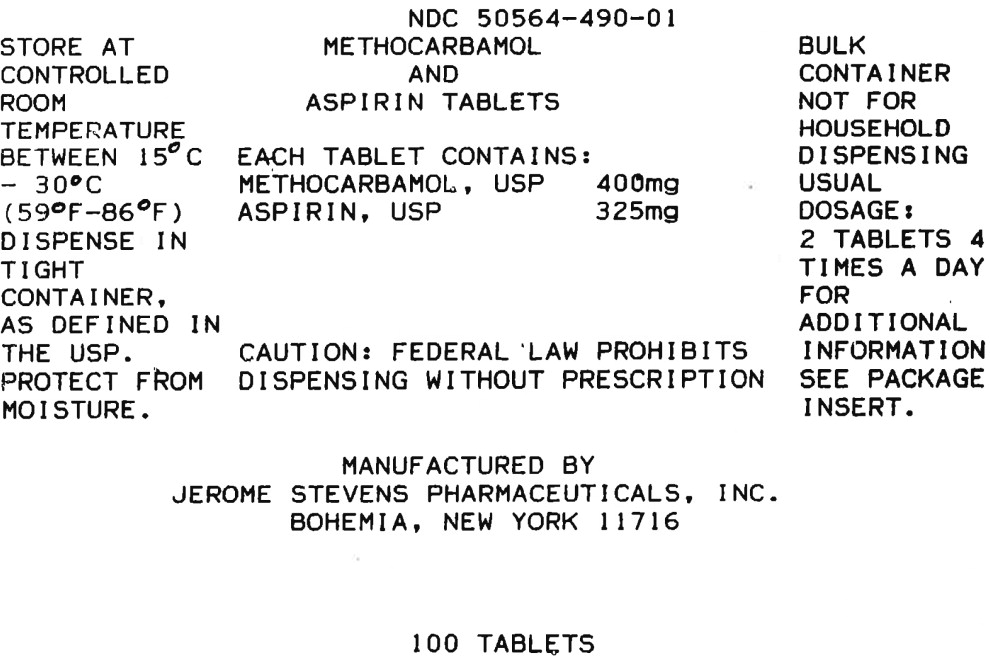
HOW SUPPLIED
Methocarbamol and Aspirin supplied as pink and white laminated, compressed tablets monogrammed JSP 490 in bottles of 100 (NDC 50564-490-01), 500 (NDC 50564-490-05).
Store at controlled room temperature 15° - 30°C (59° - 86°F). Protect from moisture.
Dispense in a tight container, as defined in the USP.
CAUTION: Federal law prohibits dispensing without prescription.
Manufactured by:
Jerome Stevens Pharmaceuticals, Inc.
Bohemia, New York 11716REV. 03/21
MG #6317 - Principal Display Panel - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
METHOCARBAMOL AND ASPIRIN
methocarbamol and aspirin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50564-490 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength methocarbamol (UNII: 125OD7737X) (methocarbamol - UNII:125OD7737X) methocarbamol 400 mg aspirin (UNII: R16CO5Y76E) (aspirin - UNII:R16CO5Y76E) aspirin 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) povidone (UNII: FZ989GH94E) croscarmellose sodium (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) silicon dioxide (UNII: ETJ7Z6XBU4) stearic acid (UNII: 4ELV7Z65AP) Product Characteristics Color pink (PINK) , white ( WHITE) Score 2 pieces Shape ROUND (ROUND) Size 13mm Flavor Imprint Code JSP490 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50564-490-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/1995 2 NDC:50564-490-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA081145 01/31/1995 Labeler - Jerome Stevens Pharmaceuticals, Inc. (021130638) Establishment Name Address ID/FEI Business Operations Jerome Stevens Pharmaceuticals, Inc. 021130638 MANUFACTURE(50564-490) , PACK(50564-490)