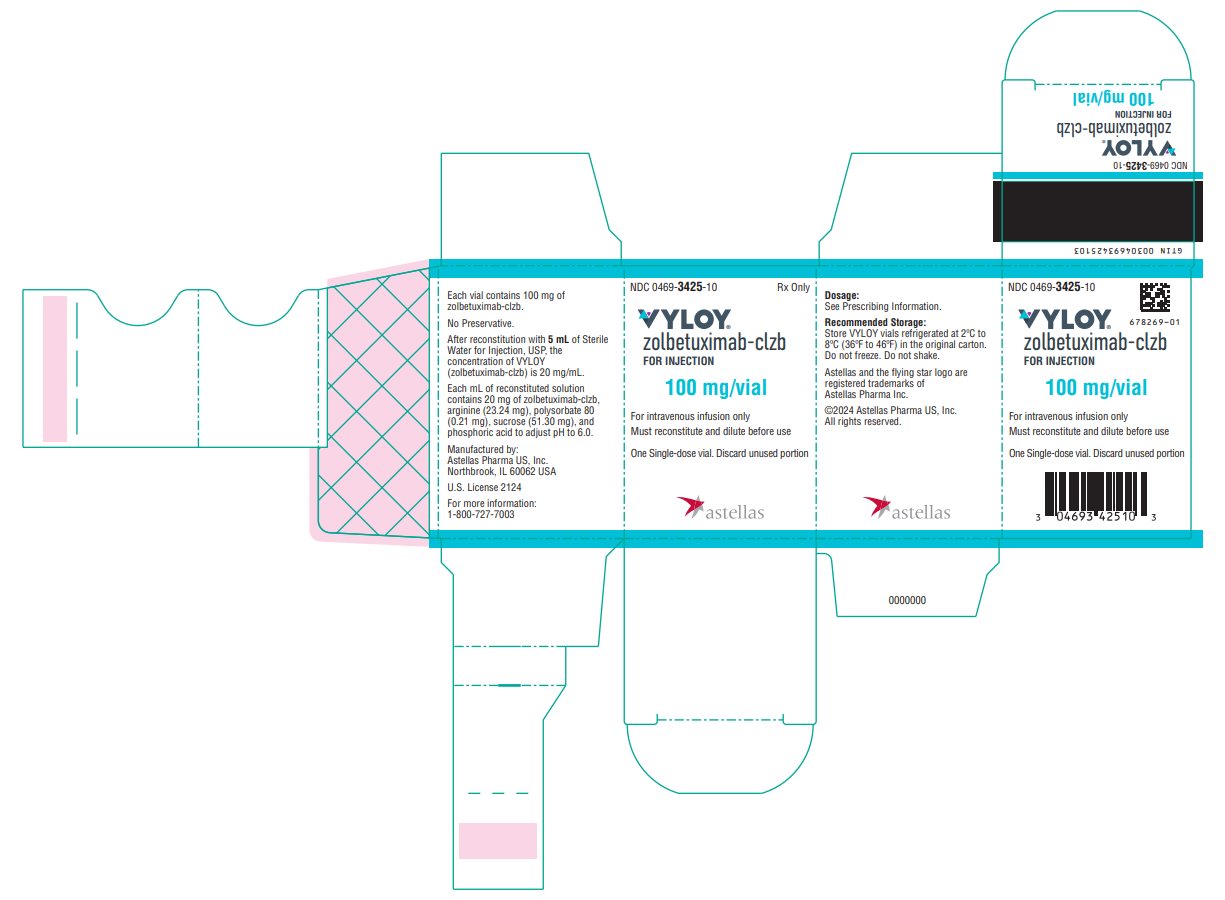
Label: VYLOY- zolbetuximab injection, powder, for suspension
- NDC Code(s): 0469-3425-10
- Packager: Astellas Pharma US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VYLOY safely and effectively. See full prescribing information for VYLOY. VYLOY® (zolbetuximab-clzb) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE VYLOY, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic human ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Patient Selection - Select adult patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumors are CLDN18.2 positive (defined as ≥75% of ...
-
3 DOSAGE FORMS AND STRENGTHS For injection: 100 mg of zolbetuximab-clzb as a white to off-white lyophilized powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity reactions, including anaphylaxis reactions, and infusion related reactions - Hypersensitivity reactions, including serious anaphylaxis reactions, and serious and fatal ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are described elsewhere in the labeling: • Hypersensitivity Reactions, including anaphylaxis, and infusion related reactions [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no data with VYLOY use in pregnant women to inform any drug-associated risks. Embryo-fetal toxicity was not observed in pregnant mice intravenously ...
-
11 DESCRIPTION Zolbetuximab-clzb is a chimeric (mouse/human) antibody composed of variable regions derived from mouse anti-human claudin-18 isoform 2 monoclonal antibody and constant regions derived from human ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Zolbetuximab-clzb is a claudin 18.2 (CLDN18.2)-directed cytolytic antibody that depletes CLDN18.2-positive cells via antibody-dependent cellular cytotoxicity (ADCC ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies in animals have been performed with zolbetuximab-clzb to evaluate carcinogenicity, mutagenicity, or impairment of ...
-
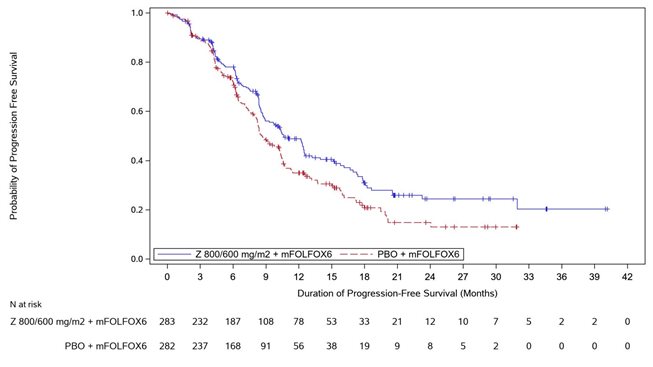
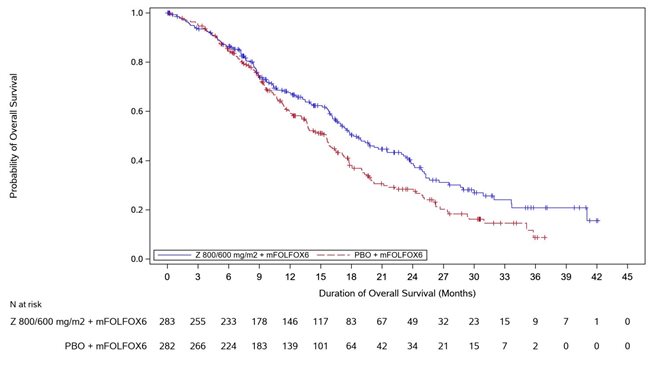
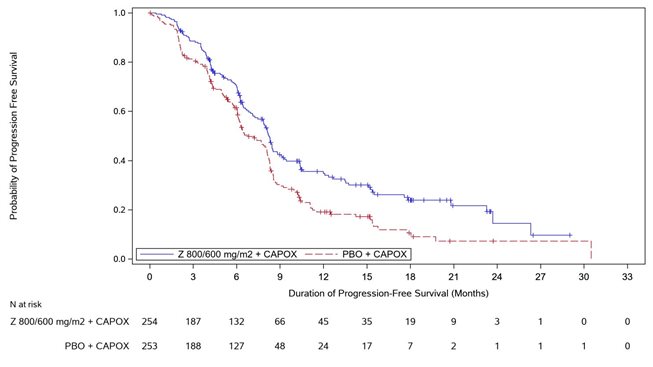
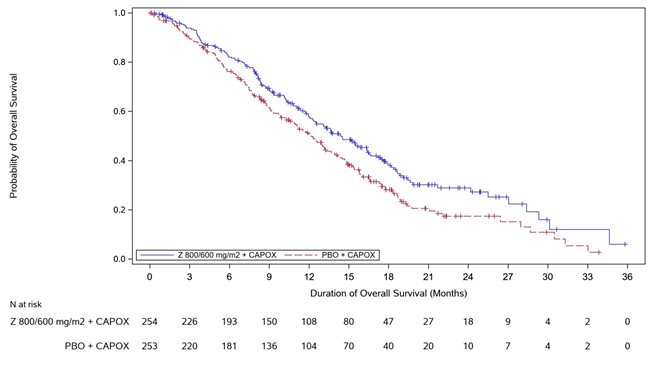
14 CLINICAL STUDIES SPOTLIGHT - The efficacy of VYLOY in combination with mFOLFOX6 was evaluated in SPOTLIGHT (NCT03504397), a double‑blind, randomized, multicenter study that enrolled 565 patients with locally ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING VYLOY (zolbetuximab-clzb) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized powder in single-dose vials. Each vial contains 100 mg of Vyloy and is available ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Hypersensitivity reactions, including anaphylaxis and infusion-related reactions - Advise patients of the risk ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - VYLOY® (vye-LOY) (zolbetuximab-clzb) for injection - What is VYLOY? VYLOY is a prescription medicine used to treat adults with cancer of the stomach ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- 100 mg/vial NDC 0469-3425-10 - Rx Only - VYLOY® zolbetuximab-clzb - FOR INJECTION - 100 mg/vial - For intravenous infusion only - Must reconstitute and dilute before use - One Single-dose vial. Discard unused ...
-
INGREDIENTS AND APPEARANCEProduct Information