Label: VAFSEO- vadadustat tablet, film coated
- NDC Code(s): 59922-641-60, 59922-642-60, 59922-643-60
- Packager: Akebia Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VAFSEO safely and effectively. See full prescribing information for VAFSEO.
VAFSEO® (vadadustat) tablets, for oral use
Initial U.S. Approval: 2024WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, and THROMBOSIS OF VASCULAR ACCESS.
See full prescribing information for complete boxed warning.
- VAFSEO increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE). (5.1)
- Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels. (5.1)
- No trial has identified a hemoglobin target level, dose of VAFSEO, or dosing strategy that does not increase these risks. (2.4)
- Use the lowest dose of VAFSEO sufficient to reduce the need for red blood cell transfusions. (2.4)
INDICATIONS AND USAGE
VAFSEO is a hypoxia-inducible factor prolyl hydroxylase (HIF PH) inhibitor indicated for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least three months. (1)
Limitations of Use
Not been shown to improve quality of life, fatigue, or patient well-being.
Not indicated for use:DOSAGE AND ADMINISTRATION
- Recommended starting dose is 300 mg orally once daily, with or without food. (2.3)
- Monitor hemoglobin levels when initiating or adjusting dose and then monthly. (2.1 and 2.4)
- Increase the dose no more frequently than once every 4 weeks. Decreases in dose can occur more frequently. (2.4)
- Adjust dose in increments of 150 mg to achieve or maintain hemoglobin levels of 10 g/dL to 11 g/dL. Doses may range from 150 mg to a maximum of 600 mg. (2.4)
DOSAGE FORMS AND STRENGTHS
- Tablets: 150 mg, 300 mg and 450 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hepatotoxicity: Has been reported in patients taking VAFSEO. Measure ALT, AST and bilirubin prior to the initiation of VAFSEO, monthly after initiation for the first 6 months, then as clinically indicated. Discontinue VAFSEO if ALT or AST is persistently elevated or accompanied by elevated bilirubin. (5.2)
- Hypertension: Worsening hypertension, including hypertensive crisis may occur. Monitor blood pressure. Adjust anti-hypertensive therapy as needed. (5.3)
- Seizures: Seizures have occurred in patients with CKD taking VAFSEO. Monitor for new-onset seizures, premonitory symptoms, or change in seizure frequency. (5.4)
- Gastrointestinal Erosion: Gastric or esophageal erosions and gastrointestinal bleeding have been reported. (5.5)
- Malignancy: May have unfavorable effects on cancer growth. Not recommended if active malignancy. (5.7)
ADVERSE REACTIONS
The most common adverse reactions (occurring at ≥ 10%) were hypertension and diarrhea. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Akebia Therapeutics, Inc. at 1-844-445-3799 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Iron supplements and iron-containing phosphate binders: Administer VAFSEO at least 1 hour before products containing iron. (7.1)
- Non-iron-containing phosphate binders: Administer VAFSEO at least 1 hour before or 2 hours after non-iron-containing phosphate binders. (7.1)
- BCRP substrates: Monitor for signs of substrate adverse reactions and consider substrate dose reduction. (7.2)
- Statins: Monitor for statin-related adverse reactions (7.2).
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, AND THROMBOSIS OF VASCULAR ACCESS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment and On-Treatment Evaluations of Anemia, Iron Stores, and Liver Tests
2.2 Important Dosing Information
2.3 Recommended Starting Dose of VAFSEO
2.4 Monitoring Response to Therapy and Dose Adjustment
2.5 Dosage Adjustments Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Death, Myocardial Infarction (MI), Stroke, Venous Thromboembolism, and Thrombosis of Vascular Access
5.2 Hepatotoxicity
5.3 Hypertension
5.4 Seizures
5.5 Gastrointestinal Erosion
5.6 Serious Adverse Reactions in Patients with Anemia Due to Chronic Kidney Disease and Not on Dialysis
5.7 Malignancy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on VAFSEO
7.2 Effect of VAFSEO on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Anemia Due to Chronic Kidney Disease in Adults on Dialysis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, AND THROMBOSIS OF VASCULAR ACCESS
VAFSEO increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE) [see Warnings and Precautions (5.1)].
Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels [see Warnings and Precautions (5.1)].
No trial has identified a hemoglobin target level, dose of VAFSEO, or dosing strategy that does not increase these risks [see Dosage and Administration (2.4)].
Use the lowest dose of VAFSEO sufficient to reduce the need for red blood cell transfusions [see Dosage and Administration (2.4)].
-
1 INDICATIONS AND USAGE
VAFSEO is indicated for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least three months.
Limitations of Use
- VAFSEO has not been shown to improve quality of life, fatigue, or patient well-being.
- VAFSEO is not indicated for use:
- As a substitute for red blood cell transfusions in patients who require immediate correction of anemia
- In patients with anemia due to CKD not on dialysis [see Warnings and Precautions (5.6)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment and On-Treatment Evaluations of Anemia, Iron Stores, and Liver Tests
Evaluation of Anemia and Iron Stores
Correct and exclude other causes of anemia (e.g., vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding) before initiation of VAFSEO. Evaluate iron status in all patients before and during treatment. Administer supplemental iron therapy when serum ferritin is less than 100 mcg/L or when serum transferrin saturation is less than 20%. The majority of patients with CKD will require supplemental iron during the course of therapy.Measure hemoglobin (Hb) at baseline and as recommended in section 2.4.
Liver Testing
Measure serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin prior to the initiation of VAFSEO and monthly after initiation for the first 6 months and then monitor as clinically indicated [see Warnings and Precautions (5.2)].Discontinue VAFSEO if there are persistent ALT or AST elevations greater than 3 times upper limit of normal (ULN) or if ALT or AST elevations greater than 3 times ULN are accompanied by a bilirubin increase greater than 2 times ULN [see Warnings and Precautions (5.2)].
2.2 Important Dosing Information
Individualize dosing and use the lowest dose of VAFSEO sufficient to reduce the need for red blood cell transfusions. Do not target a hemoglobin level higher than 11 g/dL.
VAFSEO can be taken with or without food.
VAFSEO should be swallowed whole. Tablets should not be cut, crushed, or chewed.
VAFSEO can be administered without regard to the timing or type of dialysis [see Clinical Pharmacology (12.3)].
If a dose of VAFSEO is missed, it should be taken as soon as possible, unless it is the same day as the next dose. In this case, the missed dose should be skipped, and the next dose taken at the usual time. Double doses should not be taken to make-up for a missed dose.
2.3 Recommended Starting Dose of VAFSEO
Adults Not Being Treated with an ESA
The recommended starting dose is 300 mg orally once daily.Adults Being Switched from an ESA
When converting from an ESA to VAFSEO, the recommended starting dose is 300 mg orally once daily.Taking into account the gradual rise in Hb with VAFSEO, red blood cell (RBC) transfusions or ESA treatment may be considered during the transition phase if Hb values fall below 9 g/dL or Hb response is considered not acceptable. Patients receiving RBC transfusions should continue VAFSEO treatment during the transfusion period. VAFSEO should be paused for those patients receiving temporary ESA rescue treatment and may be resumed when Hb levels are greater than or equal to 10 g/dL. Depending on the ESA used for rescue, the pause in VAFSEO treatment should be extended to:
- 2 days after the last dose of epoetin
- 7 days after the last dose of darbepoetin alfa
- 14 days after the last dose of methoxy polyethylene glycol-epoetin beta.
Following ESA rescue, VAFSEO should be resumed at the prior dose or with a dose that is 150 mg greater than the prior dose, with subsequent titration according to the dose titration guidelines given below in this section.
2.4 Monitoring Response to Therapy and Dose Adjustment
Following initiation of therapy and after each dose adjustment, monitor hemoglobin (Hb) levels, every two weeks until stable, then monitor at least monthly [see Warnings and Precautions (5.1)].
Dose Titration
Increase the dose no more frequently than once every 4 weeks. Decreases in dose can occur more frequently.Adjust dose in increments of 150 mg to achieve or maintain Hb levels within 10 g/dL to 11 g/dL. Doses may range from 150 mg to a maximum of 600 mg. When adjusting the dose, consider the patient’s Hb variability, Hb rate of rise and rate of decline, and VAFSEO responsiveness. A single Hb excursion may not require a dosing change.
- If the Hb rises rapidly (e.g., more than 1 g/dL in any 2-week period or more than 2 g/dL in 4 weeks), interrupt or reduce the dose.
- If the Hb level exceeds 11 g/dL, interrupt the dose of VAFSEO until Hb is less than or equal to 11 g/dL then resume with a dose that is 150 mg less than the dose prior to interruption.
- Treatment with VAFSEO should not be continued beyond 24 weeks of therapy if a clinically meaningful increase in Hb level is not achieved. Alternative explanations for an inadequate response should be sought and treated before re-starting therapy.
2.5 Dosage Adjustments Due to Drug Interactions
Oral Iron and Phosphate Binders
VAFSEO should be administered at least 1 hour before dosing oral iron supplements, products containing iron, or iron-containing phosphate binders [see Drug Interactions (7.1)].VAFSEO should be administered at least 1 hour before or 2 hours after dosing non-iron-containing phosphate binders [see Drug Interactions (7.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Death, Myocardial Infarction (MI), Stroke, Venous Thromboembolism, and Thrombosis of Vascular Access
VAFSEO increases the risk of arterial and venous thrombotic events, that may be fatal, including myocardial infarction, stroke, venous thromboembolism and vascular access thrombosis [see Boxed Warning, Adverse Reactions (6.1)]. Patients with cardiovascular or cerebrovascular disease are at increased risk of these events. Avoid use in patients with a history of myocardial infarction, cerebrovascular event, or acute coronary syndrome within the 3 months prior to starting VAFSEO.
A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks. Targeting a hemoglobin level of greater than 11 g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with ESAs, which also increase erythropoietin levels.
No trial has identified a hemoglobin target level, dose of VAFSEO, or dosing strategy that does not increase these risks. Use the lowest dose of VAFSEO sufficient to reduce the need for red blood cell transfusions. Adherence to dosing and hemoglobin monitoring recommendations is important to avoid excessive erythropoiesis [see Dosage and Administration (2.4)].
Advise patients to seek immediate medical attention if they develop signs or symptoms of myocardial infarction, stroke, venous thromboembolism, or thrombosis of vascular access. Evaluate and manage promptly if these occur.
5.2 Hepatotoxicity
VAFSEO may cause hepatotoxicity. In clinical trials, hepatocellular injury attributed to VAFSEO was reported in less than 1% of patients, including one case of severe hepatocellular injury with jaundice. All events were asymptomatic and resolved after discontinuation of VAFSEO. The time to onset was generally within the first 3 months of treatment.
Elevated serum ALT, AST, and bilirubin were seen in 1.8%, 1.8% and 0.3% of CKD patients treated with VAFSEO, respectively.
Measure ALT, AST and bilirubin prior to the initiation of VAFSEO and monthly after initiation for the first 6 months and then monitor as clinically indicated [see Dosage and Administration (2.1)].
Discontinue VAFSEO if there is persistent ALT or AST greater than 3 times ULN or if ALT or AST elevations greater than 3 times upper limit of normal (ULN) are accompanied by a bilirubin increase greater than 2 times ULN.
VAFSEO is not recommended in patients with cirrhosis or active, acute liver disease.
5.3 Hypertension
VAFSEO is contraindicated in patients with uncontrolled hypertension. In the INNO2VATE-1 and INNO2VATE-2 clinical trials, worsening of hypertension was reported in 14% (9.4 per 100 person-years [PY]) of patients receiving VAFSEO and 17% (11.8 per 100 PY) of patients receiving darbepoetin alfa. Serious worsening of hypertension was reported in 2.7% (1.7 per 100 PY) of patients receiving VAFSEO and 3% (1.8 per 100 PY) of patients receiving darbepoetin alfa. Cases of hypertensive crisis including hypertensive encephalopathy and seizures have also been reported in patients receiving VAFSEO. Periodically monitor blood pressure and adjust or initiate anti-hypertensive therapy as needed.
5.4 Seizures
Seizures have occurred in patients treated with VAFSEO. In the INNO2VATE-1 and INNO2VATE-2 clinical trials, seizures occurred in 1.6% (1.0 per 100 PY) of patients who received VAFSEO and 1.6% (1.0 per 100 PY) of patients who received darbepoetin alfa. Following initiation of VAFSEO, monitor patients closely for premonitory neurologic symptoms. Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms, or change in seizure frequency.
5.5 Gastrointestinal Erosion
In the INNO2VATE-1 and INNO2VATE-2 clinical trials, gastric or esophageal erosions occurred in 6.4% (4.0 per 100 PY) of patients receiving VAFSEO and 5.3% (3.3 per 100 PY) of darbepoetin alfa-treated patients. Serious gastrointestinal erosions, including gastrointestinal bleeding and the need for red blood cell transfusions were reported in 3.4% (2.1 per 100 PY) and 3.3% (2.0 per 100 PY) of those receiving VAFSEO and darbepoetin alfa, respectively. Consider this risk particularly in patients at increased risk for gastrointestinal erosions, such as those with a history of gastrointestinal erosion, peptic ulcer disease, use of concomitant medications that increase the risk of gastrointestinal erosion, and current tobacco smokers and alcohol drinkers.
Advise patients of the symptoms and signs of gastric and esophageal erosions and of gastrointestinal bleeding and to seek prompt medical care if these occur.
5.6 Serious Adverse Reactions in Patients with Anemia Due to Chronic Kidney Disease and Not on Dialysis
The safety of VAFSEO has not been established for the treatment of anemia due to CKD in adults not on dialysis and its use is not recommended in this setting [see Indications and Usage (1)].
In large clinical trials in adults with anemia of CKD who were not on dialysis (PRO2TECT-1 and PRO2TECT-2), an increased risk of mortality, stroke, myocardial infarction, serious acute kidney injury, serious hepatic injury, and serious gastrointestinal erosions was observed in patients treated with VAFSEO compared to darbepoetin alfa.
5.7 Malignancy
Because increased hypoxia inducible factor (HIF)-1 levels may be associated with unfavorable effects on cancer growth, VAFSEO has not been studied and is not recommended in patients with active malignancies. In the INNO2VATE-1 and INNO2VATE-2 clinical trials, malignancies were observed in 2.2% (1.3 per 100 PY) of patients treated with VAFSEO and 3.0% (1.8 per 100 PY) of patients treated with darbepoetin alfa. No evidence of increased carcinogenicity was observed in animal studies [see Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Increased risk of death, myocardial infarction, stroke and venous thromboembolism, and thrombosis of vascular access [see Boxed Warning and Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Seizures [see Warnings and Precautions (5.4)]
- Gastrointestinal erosion [see Warnings and Precautions (5.5)]
- Serious adverse reactions in patients with anemia due to chronic kidney disease and not on dialysis [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VAFSEO was evaluated in adults with dialysis-dependent chronic kidney disease (DD-CKD) with anemia in the INNO2VATE-1 and INNO2VATE-2 trials [see Clinical Studies (14.1)]. Both trials randomized patients to VAFSEO or darbepoetin alfa. Results in this section are based on the pooled VAFSEO treatment arms and pooled darbepoetin alfa arms from these trials.
There were 1947 patients treated with VAFSEO and 1955 patients treated with darbepoetin alfa. In the pooled VAFSEO treatment arm, 71% of the participants were treated continuously for at least 6 months of VAFSEO and 44% of participants received VAFSEO for at least 1 year.
VAFSEO was non-inferior to darbepoetin alfa on the time to first occurrence of major adverse cardiovascular events (MACE) in adults with anemia due to CKD who were on dialysis [see Clinical Studies (14.1)].
Permanent treatment discontinuation due to an adverse reaction was reported in 4.9% of patients treated with VAFSEO and 1.1% of patients treated with darbepoetin alfa. Gastrointestinal symptoms (nausea, vomiting and diarrhea) resulted in permanent treatment discontinuation in 1.8% of patients treated with VAFSEO.
The most common adverse reactions (>10% of VAFSEO-treated patients) were hypertension and diarrhea.
Table 1 lists the adverse reactions that occurred in at least 5% or greater of patients with DD-CKD treated with VAFSEO.
Table 1 Adverse Reactions (≥5%) in Patients with DD-CKD During INNO2VATE-1 and INNO2VATE-2
*Grouped Terms
Hypertension includes hypertensive crisis, pre-eclampsia and hypertensive encephalopathy.
Headache includes occipital neuralgia.
Fatigue includes asthenia, lethargy and malaise.
Vomiting includes hematemesis.
Gastrointestinal erosion includes duodenal ulcers and perforation, gastrointestinal ulcers and perforation, esophageal ulcers and perforation, and unspecified site or hematemesis, gastrointestinal hemorrhage, helicobacter duodenitis and gastritis, melaena, and gastric hemorrhage.
Dizziness includes labyrinthitis, vertigo, vestibular neuronitis and presyncope.
Dyspnea includes orthopnea and respiratory distress.Adverse Reactions VAFSEO
N=1947
(%)Darbepoetin Alfa
N=1955
(%)Hypertension* 14 17 Diarrhea* 13 10 Headache* 9 8 Nausea* 8 8 Fatigue* 8 5 Abdominal pain* 7 7 Vomiting* 7 7 Gastrointestinal erosion* 6 5 Dizziness* 6 5 Dyspnea* 6 7 Arteriovenous fistula thrombosis 6 5 Dialysis related complication 5 7 Adjudicated fatal and non-fatal thrombotic vascular events were observed in 9.0 per 100 PY of patients in the pooled VAFSEO arm and in 8.7 per 100 PY of patients in the pooled darbepoetin alfa (see Table 2).
Table 2 Adjudicated Thrombotic Vascular Events in Patients with DD-CKD (Fatal and Non-fatal Events)*
PY = Person Years *These data are not an adequate basis for comparison of rates between the study drug and active control. ** Based on time to first event analysis. Event VAFSEO
(N = 1947)Darbepoetin Alfa
(N = 1955)Rate per 100 PY** Rate per 100 PY** Vascular access thrombosis 4.8 3.9 Myocardial infarction 2.9 2.8 Stroke 1.1 1.4 Deep vein thrombosis 0.5 0.6 Pulmonary embolism 0.2 0.3 Arterial thrombosis 0.2 0.1 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on VAFSEO
Table 3 describes clinically significant drug interactions where concomitant use of another drug affects VAFSEO.
Table 3 Drug Interactions with VAFSEO that Affect Vadadustat Exposure
Iron supplements and phosphate binders Clinical Effect Co-administration with oral iron supplements, products containing iron, or phosphate binders decreases the exposure of vadadustat [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of VAFSEO. Prevention or Management Stagger administration when VAFSEO is used with oral iron supplements, products containing iron, iron-containing phosphate binders, or non-iron-containing phosphate binders [see Dosage and Administration (2.5)]. Examples* Iron supplements: ferric citrate, ferrous sulfate, sodium ferrous citrate
Iron-containing phosphate binders: ferric citrate, sucroferric oxyhydroxide
Non-iron-containing phosphate binders: calcium acetate, sevelamer carbonateOrganic anion transporter (OAT) OAT1/OAT3 inhibitors Clinical Effect Co-administration with OAT1/OAT3 (Organic Anion Transporter) inhibitors may increase the area under the concentration curve (AUC) of vadadustat [see Clinical Pharmacology (12.3)], which may increase the risk of VAFSEO adverse reactions. Prevention or Management Closely monitor for too large or too rapid an increase in Hb response and for adverse reactions. Examples* OAT1 inhibitors: probenecid, rifampicin
OAT3 inhibitors: gemfibrozil, probenecid, teriflunomide*These examples are not a comprehensive list of all possible drugs that may fit this category. 7.2 Effect of VAFSEO on Other Drugs
Table 4 describes clinically significant drug interactions where VAFSEO affects the co-administered drug.
Table 4 Drug Interactions with VAFSEO that affect co-administered drugs
BCRP substrates Clinical Effect Vadadustat may increase the exposure of BCRP substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to the BCRP substrate. Prevention or Management Monitor for signs of adverse effects of the co-administered BCRP substrate and reduce substrates’ dosage in accordance with their approved product labeling, if needed. Example* sulfasalazine Statins Clinical Effect Vadadustat increases the maximal concentration (Cmax) and AUC of some statins when co-administered [see Clinical Pharmacology (12.3)]. Prevention or
ManagementMonitor for possible statin-related adverse reactions. Concomitant Drug Name Recommendation Simvastatin Starting dose of simvastatin should be 5 mg/day. Maximum daily dose of simvastatin not to exceed 20 mg. Rosuvastatin Maximum daily dose of rosuvastatin not to exceed 5 mg. OAT3 substrates Clinical Effect Vadadustat may increase the exposure of co-administered OAT3 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates. Prevention or Management Monitor for signs of adverse reactions of the co-administered OAT3 substrates and adjust substrates’ dosage in accordance with their approved product labeling, if needed. Examples* cefaclor, ceftizoxime, famotidine, furosemide, oseltamivir carboxylate, penicillin G, sitagliptin *These examples are not a comprehensive list of all possible drugs that may fit this category. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with VAFSEO use in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with CKD (see Clinical Considerations). Vadadustat administration orally to pregnant rats and rabbits during the period of organogenesis was associated with reduced fetal weight at doses that caused maternal toxicity. In rat and rabbit studies, vadadustat was not teratogenic (see Data).The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. VAFSEO should only be used during pregnancy if the benefit justifies the potential risk to the fetus.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: CKD in pregnancy increases the risk for maternal hypertension, preeclampsia, miscarriage, stillbirth, preterm delivery, low birth weight infants, and polyhydramnios.Data
Animal Data
Vadadustat decreased fetal weight and reduced fetal skeletal ossification in rats at a dose of 160 mg/kg/day (1.7 times the maximum recommended human dose [MRHD] based on AUC), which was associated with maternal toxicity defined by reduced body weight gain and food consumption.Vadadustat was orally administered to pregnant rabbits at doses of 10, 25, or 50 mg/kg/day from gestation day 6 until gestation day 18 during the period of organogenesis. Vadadustat administration at 50 mg/kg/day resulted in maternal toxicity of reduced body weight gain, but no adverse effects on embryofetal development were observed at doses less than or equal to 50 mg/kg/day (1.5 times the MRHD based on AUC).
In a pre- and postnatal development study, pregnant rats were dosed orally with vadadustat 20, 40, or 80 mg/kg/day from implantation until weaning (gestation day 6 to lactation day 20) at 20, 40, or 80 mg/kg/day. There were decreased body weights of offspring at the dose of 80 mg/kg/day but no adverse effects were observed at doses less than or equal to 80 mg/kg/day (0.3 times the MRHD based on AUC) in dams.
8.2 Lactation
Risk Summary
There are no data on the presence of vadadustat in human milk, the effects of vadadustat on the breastfed child, or the effects on milk production. Vadadustat is present in the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Given the serious adverse reactions seen in adults treated with VAFSEO, such as thrombotic vascular events, advise patients not to breastfeed during treatment with VAFSEO, and for 2 days after the final dose.Data
Vadadustat was detected in the milk of lactating rats after a single oral administration of radiolabeled vadadustat at 50 mg/kg. The maximum ratio of milk to plasma concentration in rats was 14.5 at 8 hours postdose and the ratio of milk to plasma AUC was 6.8.4 Pediatric Use
The safety and effectiveness of VAFSEO in pediatric patients have not been established.
8.5 Geriatric Use
There were 1330 patients 65 years of age and older in the pooled INNO2VATE-1 and INNO2VATE-2 clinical trials. Of the total number of VAFSEO-treated patients in these studies, 449 (23%) were 65 to 74 years of age, 194 (10%) were 75 to 84 years of age, and 24 (1%) were 85 years of age and older. No overall differences in safety or effectiveness were observed between patients 65 years of age and older and younger adult patients [see Clinical Studies (14.2)].
8.6 Hepatic Impairment
VAFSEO is not recommended in patients with cirrhosis or active, acute liver disease [see Warnings and Precautions (5.2)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Drug abuse is intentional non-therapeutic use of a drug, even once, for its rewarding psychological or physiological effects. Abuse of VAFSEO may be seen in athletes for the effects on erythropoiesis.
Abuse-Related Adverse Reactions
There are no data on the abuse of VAFSEO in humans. Vadadustat and its metabolites neither selectively penetrate the central nervous system, nor produce behavioral effects in animals that are consistent with central nervous system activity.
Misuse of drugs that increase erythropoiesis, such as VAFSEO, by healthy persons may lead to polycythemia, which may be associated with life-threatening cardiovascular complications (e.g., stroke, myocardial infarction, and thromboembolism).
-
10 OVERDOSAGE
VAFSEO overdose may result in exaggeration of the pharmacologic effects such as increased Hb. VAFSEO overdose should be managed as clinically appropriate (e.g., reduction of VAFSEO dose or discontinuation). Approximately 16% of the vadadustat dose is removed by dialysis. There is no specific antidote.
-
11 DESCRIPTION
VAFSEO contains vadadustat, a hypoxia-inducible factor prolyl hydroxylase (HIF PH) inhibitor. Vadadustat is 2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic acid. Vadadustat has a molecular weight of 306.70. The empirical formula is C14H11ClN2O4.
The chemical structure is:
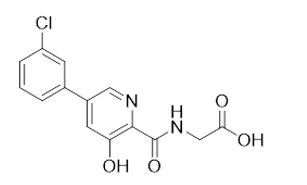
Vadadustat is a white to off-white solid that is practically insoluble in water.
Vadadustat is formulated as a film-coated, immediate-release tablet for oral administration.
VAFSEO is available in 150 mg, 300 mg and 450 mg strengths. Inactive ingredients include: colloidal silicon dioxide, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The tablet film-coating contains polyvinyl alcohol, polyethylene glycol (PEG) and talc.
Colorants include:
150 mg tablet - titanium dioxide
300 mg tablet - titanium dioxide and yellow iron oxide
450 mg tablet - titanium dioxide, iron oxide red and ferrosoferric oxide -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vadadustat is a reversible inhibitor of HIF-prolyl-4-hydroxylases (PH)1, PH2, and PH3 (IC50 in the nM range). This activity results in the stabilization and nuclear accumulation of HIF-1α and HIF-2α transcription factors, and increased production of erythropoietin (EPO).
12.2 Pharmacodynamics
After a single dose of vadadustat 80 to 1200 mg (0.27 to 4 times the approved recommended starting dosage) in healthy male adults, a dose-dependent increase in EPO was observed.
Cardiac Electrophysiology
At a dose of 600 mg or 1200 mg (2 or 4 times the approved recommended starting dosage), VAFSEO does not prolong the QTc interval to any clinically relevant extent.12.3 Pharmacokinetics
Vadadustat AUC and observed peak concentration (Cmax) increased proportionally after single doses from 80 mg to 1200 mg (0.27 to 4 times the approved recommended starting dosage). Vadadustat is expected to reach steady state by day 3 following once daily dosing, with no significant accumulation.
Absorption
The time to peak plasma concentration (Tmax) of vadadustat is approximately 2 to 3 hours.Effect of Food
No clinically significant differences in vadadustat pharmacokinetics were observed following administration of a high-fat meal.Distribution
Protein binding of vadadustat is ≥99.5% in human plasma. Vadadustat does not distribute into red blood cells.Elimination
The mean half-life of vadadustat in patients on chronic hemodialysis was 9.2 hours.Metabolism
Vadadustat is primarily metabolized via glucuronidation by UDP-glucuronosyltransferase (UGT) enzymes.Excretion
After a single radiolabeled oral dose to healthy adults, 85.9% of the total dose was recovered; 58.9% in urine (<1% of total dose unchanged); and 26.9% in feces (9% of total dose unchanged).Specific Populations
There were no clinically significant differences in the pharmacokinetics of vadadustat based on age, sex, race/ethnicity, or moderate hepatic impairment (Child-Pugh Class B). The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of vadadustat is unknown.Patients with Renal Impairment
Vadadustat clearance decreased with decreasing renal function and exposures in DD-CKD were approximately 2-fold higher compared to healthy adults. In patients with Stage 5 DD-CKD, no significant differences in pharmacokinetics (Cmax, AUC or mean half-life) were observed when VAFSEO was administered 4 hours before dialysis or 2 hours after dialysis.Drug Interaction Studies
The impact of co-administered drugs on vadadustat exposure was examined in several drug-drug interaction (DDI) studies in healthy adults. The change in vadadustat exposure with coadministration compared to VAFSEO alone is summarized in Figure 1.Figure 1 Impact of Other Drugs on Pharmacokinetics of Vadadustat
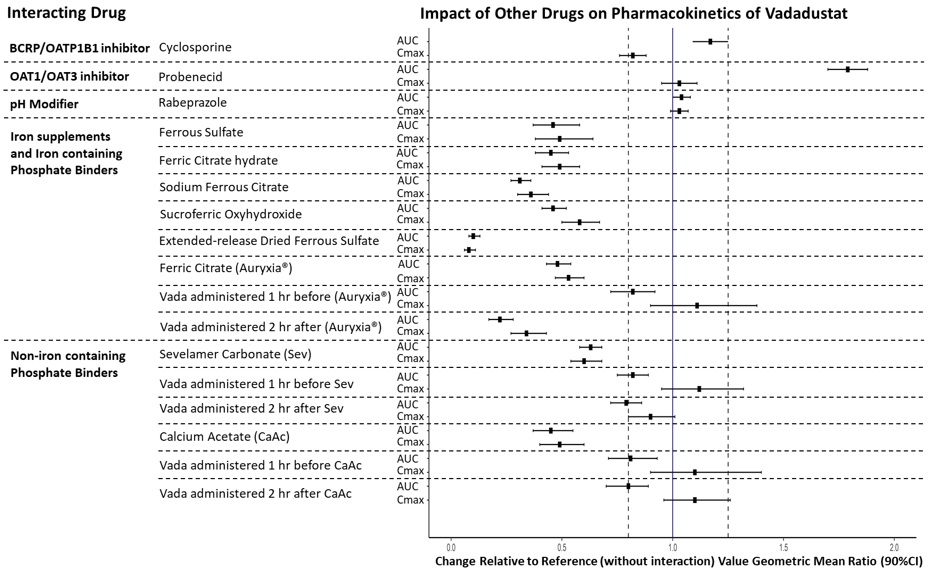
BCRP: breast cancer resistance protein; CI: confidence interval; OAT: organic anion transporter; OATP: organic anion-transporting polypeptide. The solid vertical line represents geometric mean ratio of 1 and dotted vertical lines represent the 0.80 to 1.25 boundary.
The impact of vadadustat on the exposure of other drugs was examined in several DDI studies in healthy adults. The change in drug exposure when coadministered with VAFSEO compared to the drug alone is summarized in Figure 2.
Figure 2 Effect of Vadadustat on Pharmacokinetics of Other Drugs
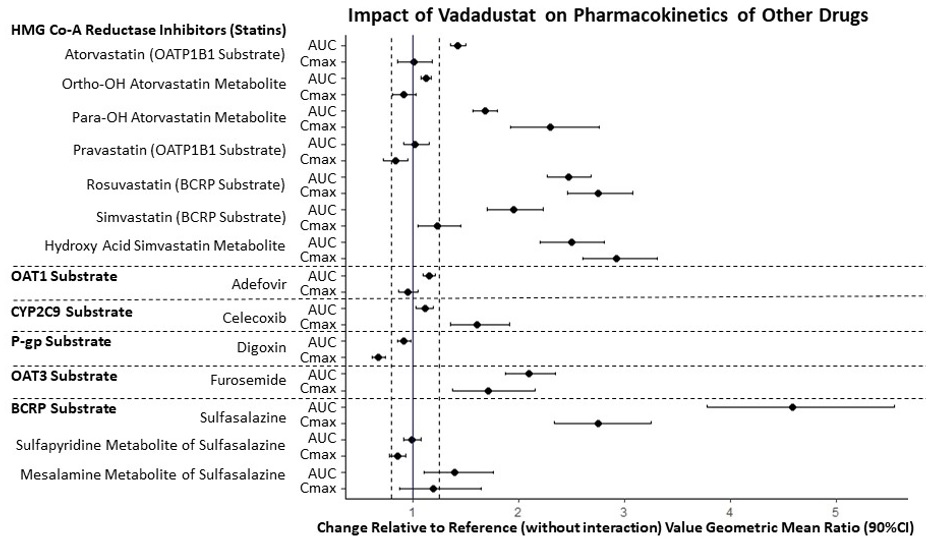
BCRP: breast cancer resistance protein; CI: confidence interval; OAT: organic anion transporter; OATP: organic anion-transporting polypeptide. The solid vertical line represents geometric mean ratio of 1 and dotted vertical lines represent the 0.80 to 1.25 boundary.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Vadadustat was not carcinogenic when administered orally at doses of 2, 7, and 20 mg/kg/day in a 6-month study in transgenic mice and at doses of 5, 15, and 50 mg/kg/day in a 2-year study in rats. The highest exposure to vadadustat in rats corresponds to 0.2 times the MRHD based on AUC.
Vadadustat was negative for mutagenicity in the in vitro bacterial reverse mutation assay. Vadadustat exhibited clastogenic activity in vitro but was negative in the in vivo chromosomal aberration assay in peripheral blood lymphocytes and comet assay in rats. Based on the weight of evidence, vadadustat is not considered genotoxic.
Fertility and early embryonic development toxicity studies were conducted in rats at dose levels of 40 to 120 mg/kg/day. Vadadustat did not impact fertility or reproduction in rats up to 80 mg/kg/day (1.5 times the MRHD based on AUC).
-
14 CLINICAL STUDIES
14.1 Treatment of Anemia Due to Chronic Kidney Disease in Adults on Dialysis
The efficacy and safety of VAFSEO given once daily for the treatment of anemia in adults with CKD on dialysis were demonstrated in two global, multi-center, randomized, active-controlled, non-inferiority, open-label trials in a total of 3923 patients with DD-CKD (INNO2VATE-1 and INNO2VATE-2). Patients in each trial were randomized 1:1 to receive VAFSEO with a starting dose of 300 mg once daily or darbepoetin alfa administered subcutaneously or intravenously as per the prescribing information for 52 weeks to assess the efficacy endpoints. VAFSEO was titrated in increments of 150 mg up to 600 mg to achieve the Hb target. After 52 weeks, patients continued study medication to assess long-term safety until the event-driven major adverse cardiovascular event (MACE) endpoints were reached. Efficacy in each study was based on the difference in mean change of Hb from baseline to the primary evaluation period (Weeks 24 to 36). An additional efficacy endpoint was the difference in mean change of Hb from baseline to the secondary evaluation period (Weeks 40 to 52). MACE was defined as all-cause mortality, non-fatal MI and non-fatal stroke and was evaluated in both trials.
The baseline Hb values were between 8 to 11 g/dL in the United States (US) and 9 to 12 g/dL outside the US. INNO2VATE-1 (NCT02865850) included patients with incident DD-CKD who initiated dialysis within 16 weeks prior to the beginning their trial participation and who were ESA-naive, had limited prior ESA use or were maintained on ESAs. INNO2VATE-2 (NCT02892149) included patients on chronic maintenance dialysis for more than 12 weeks who had converted from prior ESA therapy. In INNO2VATE-1, INNO2VATE-2, and the pooled INNO2VATE program the median and range of time from initiating dialysis to starting vadadustat was 0.1 (0.01 to 0.4), 2.7 (0.2 to 31.3) and 2.3 (0.01 to 31.3) years, respectively. The pooled population from the two trials had a range of 19 to 93 years of age, 55.9% were male, and the percentage of Caucasian, Hispanic, Black (including African Americans) and Asian patients was 64.5%, 38.5%, 24.1% and 4.5%, respectively. In both trials, VAFSEO was non-inferior to darbepoetin alfa in correcting and maintaining Hb levels across geographic-specific target Hb ranges [10 to 11 g/dL in the US and 10 to 12 g/dL outside the US] in adults with DD-CKD at weeks 24 to 36 and weeks 40 to 52.
Efficacy results are provided in Table 5. There were no apparent differences in response to VAFSEO across subgroups of age, gender, race and region.
Table 5 VAFSEO EFFICACY RESULTS: INNO2VATE TRIALS
CI: confidence interval; LSM: least squares mean; SD: standard deviation
A pre-specified non-inferiority margin of -0.75 g/dL was used to determine efficacy of VAFSEO.
The estimated treatment difference (VAFSEO – Darbepoetin Alfa) is obtained from an analysis of covariance (ANCOVA) model (treatment group, baseline Hb level, stratification factors [region and NYHA-CHF] as predictor variables) with multiple imputation.INNO2VATE 1 INNO2VATE 2 Hemoglobin (g/dL) VAFSEO
N = 181Darbepoetin
Alfa
N = 188VAFSEO
N = 1777Darbepoetin
Alfa
N = 1777Baseline Mean (SD) 9.4 (1.1) 9.2 (1.1) 10.3 (0.9) 10.2 (0.8) Week 24 to 36 Mean (SD) 10.4 (1.1) 10.6 (0.9) 10.4 (1.0) 10.5 (1.0) Adjusted LSM change from baseline [95% CI] 1.3 [1.1, 1.5] 1.6 [1.4, 1.8] 0.2 [0.1, 0.3] 0.4 [0.3, 0.4] Treatment Difference [95% CI] VAFSEO – Darbepoetin Alfa -0.3 [-0.5, -0.1] -0.2 [-0.2, -0.1] Week 40 to 52 Mean (SD) 10.5 (1.2) 10.6 (1.1) 10.4 (1.0) 10.6 (1.0) Adjusted LSM Change from Baseline [95% CI] 1.4 [1.2, 1.7] 1.5 [1.2, 1.8] 0.2 [0.2, 0.3] 0.4 [0.3, 0.5] Treatment Difference [95% CI] VAFSEO – Darbepoetin Alfa -0.1 [-0.3, 0.2] -0.2 [-0.3, -0.1] Cardiovascular Outcomes - Patients with Dialysis-Dependent CKD
MACE was assessed in a combined analysis of INNO2VATE-1 and INNO2VATE-2. VAFSEO was non-inferior to darbepoetin alfa in time to first occurrence of MACE (Hazard Ratio 0.96; 95% CI 0.83, 1.11). Non-inferiority of VAFSEO was established because the upper bound of the 95% CI for the MACE hazard ratio was less than the pre-specified non-inferiority margin of 1.25. The results were consistent for the individual components of the MACE endpoint (see Table 6).Table 6 Major Adverse Cardiovascular Events in the INNO2VATE Trials (ITT Analysesa)
CI = Confidence interval; ITT = Intent to treat; MACE = Major adverse cardiovascular events; PY = Person Years
aITT analyses included events on and off treatment after randomization in patients who received at least one dose of study medication.
bAdjusted for baseline covariatesVAFSEO
N = 1947
PY=3134.4Darbepoetin Alfa
N = 1955
PY=3164.0First occurrence of MACE 355 377 All-cause Mortality, n 253 253 Non-fatal Myocardial Infarction, n 76 87 Non-fatal Stroke, n 26 37 MACE Hazard Ratiob (95% CI) 0.96 (0.83, 1.11) MACE Incidence Rate per 100 PY 11.3 11.9 An analysis of the US region (N=2361 of 3902 total patients globally) for MACE and the individual MACE components, where patients were treated to a Hb target of 10 to 11 g/dL, showed a similar risk of MACE compared to darbepoetin alfa. The results were consistent for the individual components of the MACE endpoint (see Table 7).
Table 7 INNO2VATE Analyses of the MACE endpoint and the individual components for the US region where the patients were treated to a Hb target of 10 to 11 g/dL
CI = Confidence interval; ITT = Intent to treat; MACE = Major adverse cardiovascular events; PY = Person Years
aAdjusted for baseline covariatesVAFSEO
N = 1180
PY=2119.6Darbepoetin Alfa
N = 1181
PY=2133.7First occurrence of MACE 265 269 All-cause Mortality 179 174 Non-fatal Myocardial Infarction 68 69 Non-fatal Stroke 18 26 MACE Hazard Ratioa (95% CI) 1.00 (0.84, 1.18) MACE Incidence rate per 100 PY 12.5 12.6 -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
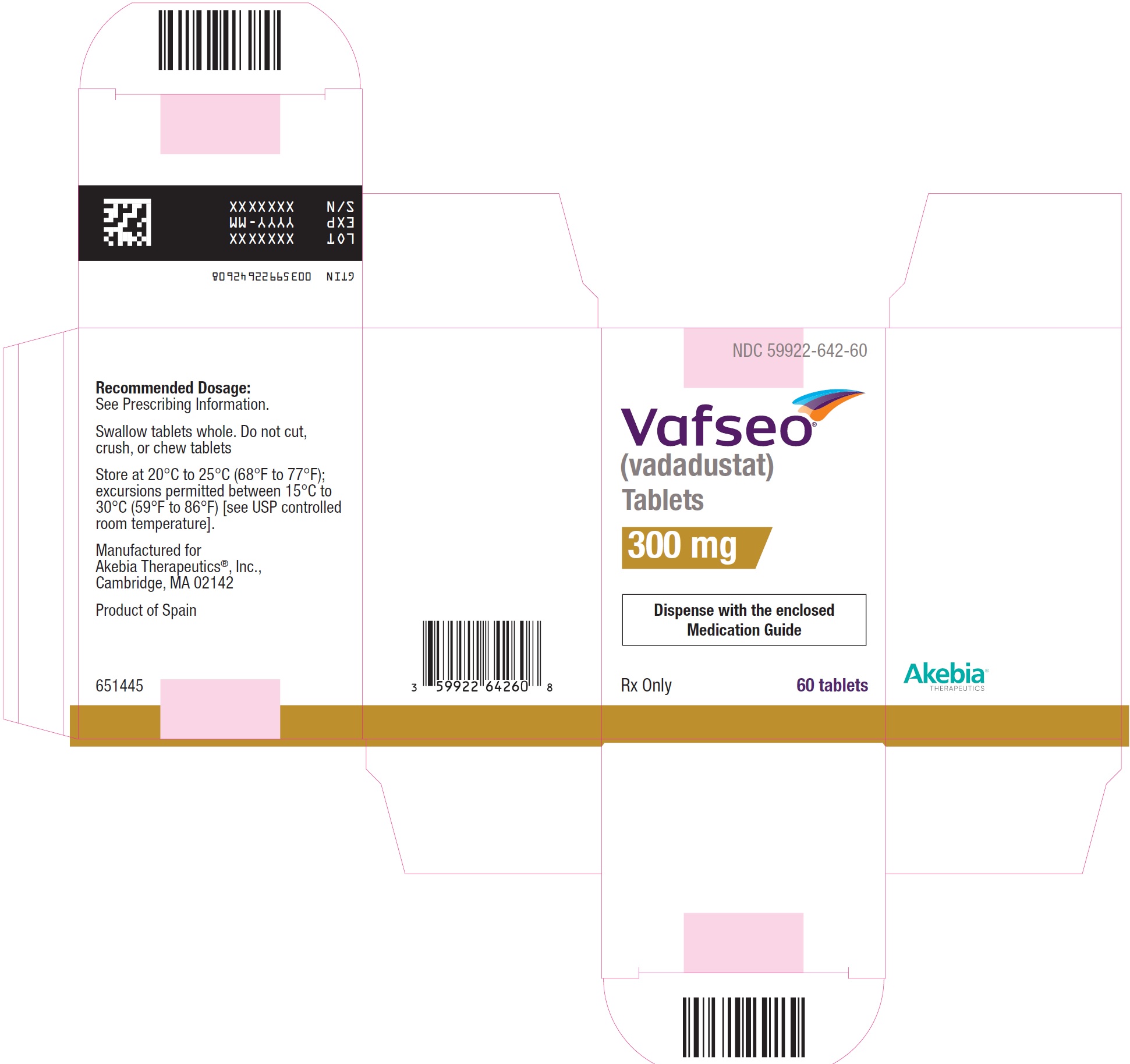
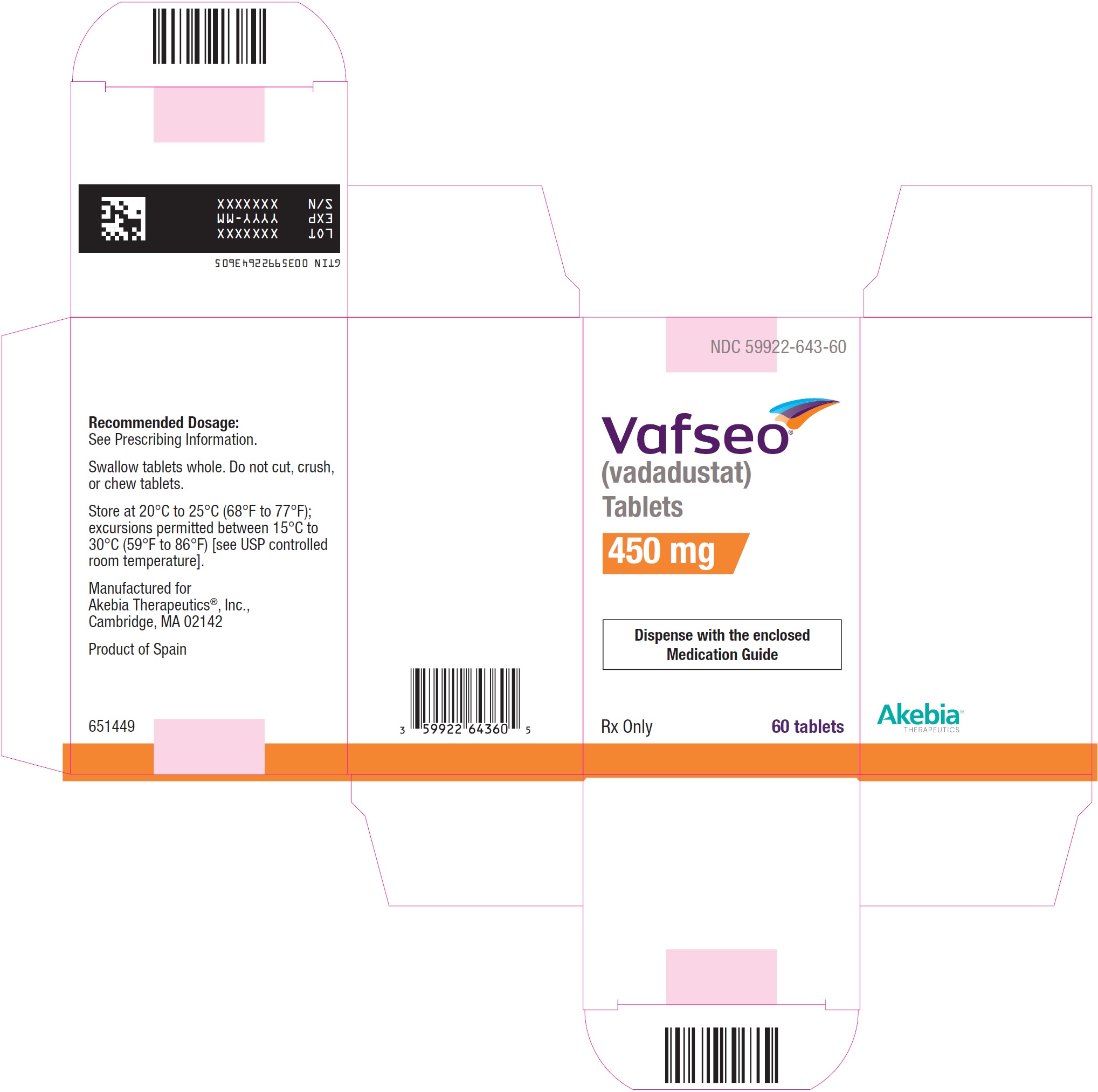
VAFSEO film-coated tablets are available in the following strengths and packages:Tablet
StrengthTablet
Shape/ColorTablet Markings Pack size NDC 150 mg Round/white “VDT” and “150” 60 count bottle 59922-641-60 300 mg Oval/yellow “VDT” and “300” 60 count bottle 59922-642-60 450 mg Oval/pink “VDT” and “450” 60 count bottle 59922-643-60 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients:
Of the increased risk of death, myocardial infarction, stroke, and thromboembolism including vascular access thrombosis [see Warnings and Precautions (5.1)].
That hemoglobin levels will be monitored when initiating or adjusting therapy, every two weeks until stable, then at least monthly [see Warnings and Precautions (5.1)].
Of the risk of hepatotoxicity and that liver tests will be measured prior to the initiation of VAFSEO, monthly after initiation for the first 6 months, then as clinically indicated [see Warnings and Precautions (5.2)].
Of the risk of hypertension and advise patients of the importance to comply with antihypertensive therapy and monitoring of blood pressure [see Warnings and Precautions (5.3)].
Of the risk of seizures and advise patients to contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency [see Warnings and Precautions (5.4)].
Of the risk of gastrointestinal erosions and advise patients to contact their healthcare provider for signs and symptoms of gastric and esophageal erosions and of gastrointestinal bleeding [see Warnings and Precautions (5.5)].
Manufactured for: Akebia Therapeutics®, Inc. Cambridge, MA 02142
VAFSEO® is a registered trademark of Akebia Therapeutics, Inc.
©2024 Akebia Therapeutics, Inc. All rights reserved.
Version No. 1
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 03/2024 MEDICATION GUIDE
VAFSEO® (VAFF-see-oh)
(vadadustat)
tablets, for oral useWhat is the most important information I should know about VAFSEO?
VAFSEO may cause serious side effects, including:
-
Increased risk of death, heart attack, stroke, and blood clots. These risks may happen if you are treated with VAFSEO to increase red blood cells (RBCs) to near the same level found in healthy people. These risks may be increased if you have heart or blood vessel problems, or problems with blood flow to your brain (cerebrovascular disease). Blood clots can form in the blood vessels (veins), including your legs (deep vein thrombosis or DVT), lungs (pulmonary embolism or PE), and in your dialysis access (vascular access thrombosis or VAT).
Get medical help right away if you get any of the following signs or symptoms:- chest pain
- trouble breathing or shortness of breath
- cold sweat
- sudden numbness or weakness in your face, arm or leg, especially on one side of your body
- sudden confusion, trouble speaking, or trouble understanding others’ speech
- sudden trouble seeing
- sudden trouble walking, dizziness, loss of balance or coordination
- loss of consciousness (fainting)
- severe headache
- pain in your legs, with or without swelling
- a cool or pale arm or leg
- changes to your dialysis access site, including swelling or discoloration near the site
- do not feel a vibration (“thrill”) over the dialysis access area
See “What are the possible side effects of VAFSEO?” for more information about side effects.
If you decide to take VAFSEO, your healthcare provider should prescribe the lowest dose of VAFSEO that is necessary to reduce your chance of needing red blood cell transfusions.What is VAFSEO?
VAFSEO is a prescription medicine used to treat anemia caused by chronic kidney disease (CKD) in adults on dialysis for at least 3 months. People with anemia have a lower-than-normal number of red blood cells (RBCs). VAFSEO works by increasing a protein called erythropoietin to help your body make more RBCs. VAFSEO is used to reduce or avoid the need for RBC transfusions.
If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take VAFSEO, even if you do not have an increase in your hemoglobin levels.
VAFSEO has not been proven to improve quality of life, tiredness (fatigue), or well-being.VAFSEO should not be used:
- in place of emergency treatment for anemia (red blood cell transfusions).
- for the treatment of anemia that is caused by CKD in people who are not on dialysis.
It is not known if VAFSEO is safe and effective in children.
Who should not take VAFSEO?
Do not take VAFSEO if you:
- are allergic to VAFSEO or to any of its ingredients. See the end of this Medication Guide for a complete list of ingredients in VAFSEO.
- have high blood pressure that is not controlled (uncontrolled hypertension).
Before taking VAFSEO, tell you healthcare provider about all of your medical conditions, including if you:
- have heart disease.
- have had a stroke.
- have liver problems.
- have high blood pressure.
- have had a seizure.
- have a history of damage, such as ulcers, to the lining of the esophagus, stomach, or intestine.
- smoke tobacco or drink alcohol.
- have cancer.
- are pregnant or plan to become pregnant. It is not known if VAFSEO may harm your unborn baby. Talk to your healthcare provider if you become pregnant or think you may be pregnant during treatment with VAFSEO.
- are breastfeeding or plan to breastfeed. It is not known if VAFSEO passes into breast milk. Do not breastfeed during treatment with VAFSEO and for 2 days after your final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- Especially tell your healthcare provider if you take iron supplements, products containing iron or phosphate binders by mouth. These products affect how VAFSEO works and should not be taken at the same time you take VAFSEO. Ask your healthcare provider if you are not sure if you take any of these products. See “How should I take VAFSEO?” for more information.
- Take VAFSEO exactly as your healthcare provider tells you to take it.
- Do not change your dose unless your healthcare provider tells you to.
- You may take VAFSEO with or without food.
- VAFSEO tablets should be swallowed whole. Tablets should not be cut, crushed, or chewed.
- VAFSEO can be taken at any time of the day, including before, during, or after dialysis.
- Take VAFSEO at least 1 hour before you take any of the following:
- iron supplements
- products containing iron
- phosphate binders that contain iron
- Take VAFSEO at least 1 hour before or 2 hours after taking the following:
- phosphate binders that do not contain iron
- If you miss a dose of VAFSEO, take it as soon as you remember during the same day and then take the next dose at the usual time the next day. Do not take more than 1 dose a day.
- Tell your healthcare provider right away if you take more than your prescribed dose of VAFSEO.
- Your healthcare provider will do certain blood tests before you start VAFSEO and during treatment as needed.
- Your healthcare provider may change your dose of VAFSEO based on the results of your blood tests.
What are the possible side effects of VAFSEO?
VAFSEO may cause serious side effects, including:
- See “What is the most important information I should know about VAFSEO?”
-
Liver problems. VAFSEO may cause changes in blood liver tests, which may be a sign of liver injury. Your healthcare provider will do blood liver tests before starting treatment with VAFSEO, monthly for the first 6 months and then as needed. Your healthcare provider may stop treatment with VAFSEO if you have changes in your blood liver tests. Tell your healthcare provider if you get any of these symptoms:
- tiredness
- pain in your right upper stomach area (abdomen)
- yellowing of your skin or the white part of your eyes
- loss of appetite
- dark urine
- High blood pressure. VAFSEO may cause you to develop new or worsening high blood pressure. It is important to check your blood pressure regularly and to follow any instructions from your healthcare provider about how to control your blood pressure. Tell your healthcare provider about any changes in your blood pressure.
- Seizures. Seizures have happened in people treated with VAFSEO. Tell your healthcare provider about new onset of seizures, change in how often you have seizures, or if you get any of the following signs or symptoms that a seizure may happen: headache, irritability, fear, confusion, or unusual feelings.
-
Damage, such as ulcers, to the lining of the esophagus, stomach, or intestines (gastrointestinal erosion). Your risk of gastrointestinal erosion may increase if you have a history of gastrointestinal erosion, stomach ulcers (peptic ulcer disease), use certain medicines that increase the risk of gastrointestinal erosion, or currently smoke tobacco or drink alcohol. Get medical care right away if you get any of these signs or symptoms:
- stomach-area (abdominal) discomfort or pain
- black, tarry stools
- nausea or vomiting
- trouble swallowing
- blood in your vomit or stool
- pain in your throat or chest
- Cancer. Cancer was observed in people treated with VAFSEO. Talk to your healthcare provider if you have any concerns about cancer.
The most common side effects of VAFSEO include:
- high blood pressure
- diarrhea
These are not all the possible side effects of VAFSEO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VAFSEO?
- Store VAFSEO at room temperature between 68℉ to 77℉ (20℃ to 25℃).
Keep VAFSEO and all medicines out of the reach of children.
General information about the safe and effective use of VAFSEO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VAFSEO for a condition for which it was not prescribed. Do not give VAFSEO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VAFSEO that is written for health professionals.
What are the ingredients in VAFSEO?
Active ingredient: vadadustat.
Inactive ingredients: colloidal silicon dioxide, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
The tablet film-coating contains polyvinyl alcohol, polyethylene glycol (PEG) and talc.
Colorants include:
150 mg tablets: titanium dioxide
300 mg tablets: titanium dioxide and yellow iron oxide
450 mg tablets: titanium dioxide, iron oxide red and ferrosoferric oxide
Manufactured for: Akebia Therapeutics®, Inc., Cambridge, MA 02142
VAFSEO® is a registered trademark of Akebia Therapeutics, Inc.
©2024 Akebia Therapeutics, Inc. All rights reserved.
For more information, go to www.VAFSEO.com or call 1-844-445-3799.
v1
-
Increased risk of death, heart attack, stroke, and blood clots. These risks may happen if you are treated with VAFSEO to increase red blood cells (RBCs) to near the same level found in healthy people. These risks may be increased if you have heart or blood vessel problems, or problems with blood flow to your brain (cerebrovascular disease). Blood clots can form in the blood vessels (veins), including your legs (deep vein thrombosis or DVT), lungs (pulmonary embolism or PE), and in your dialysis access (vascular access thrombosis or VAT).
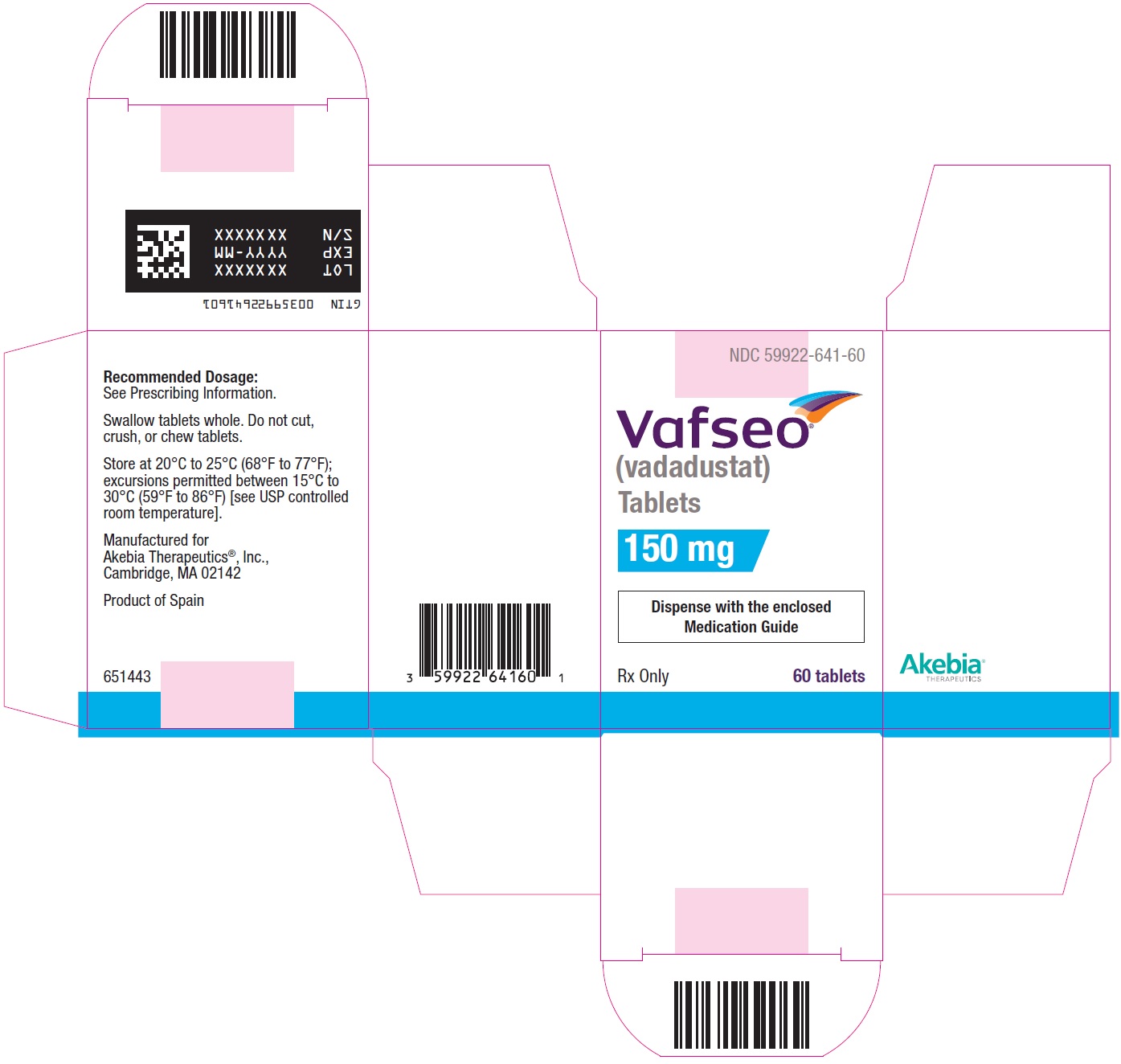
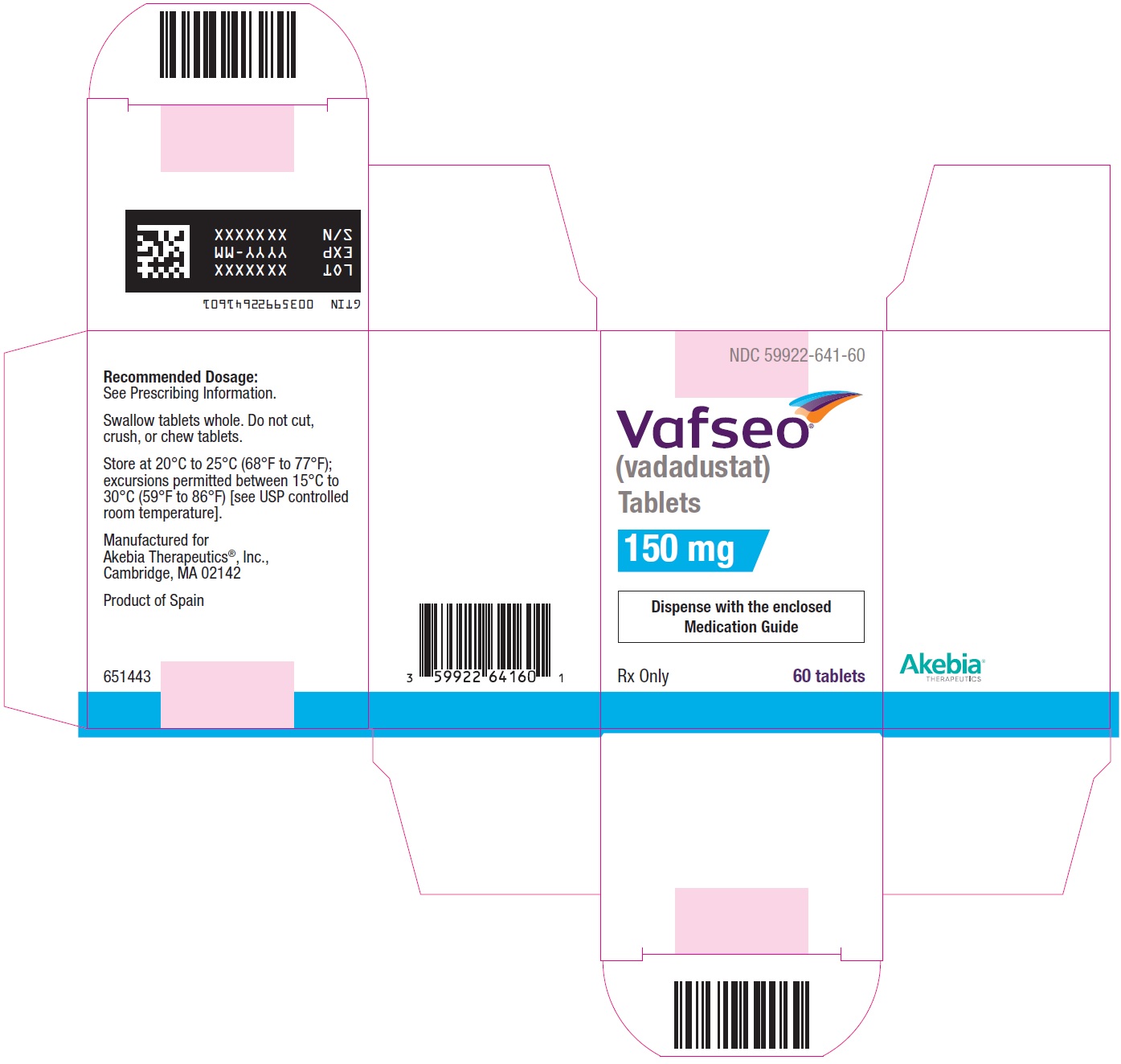
- PRINCIPAL DISPLAY PANEL - NDC: 59922-641-60 - 150 mg Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 59922-641-60 - 150 mg Bottle Label
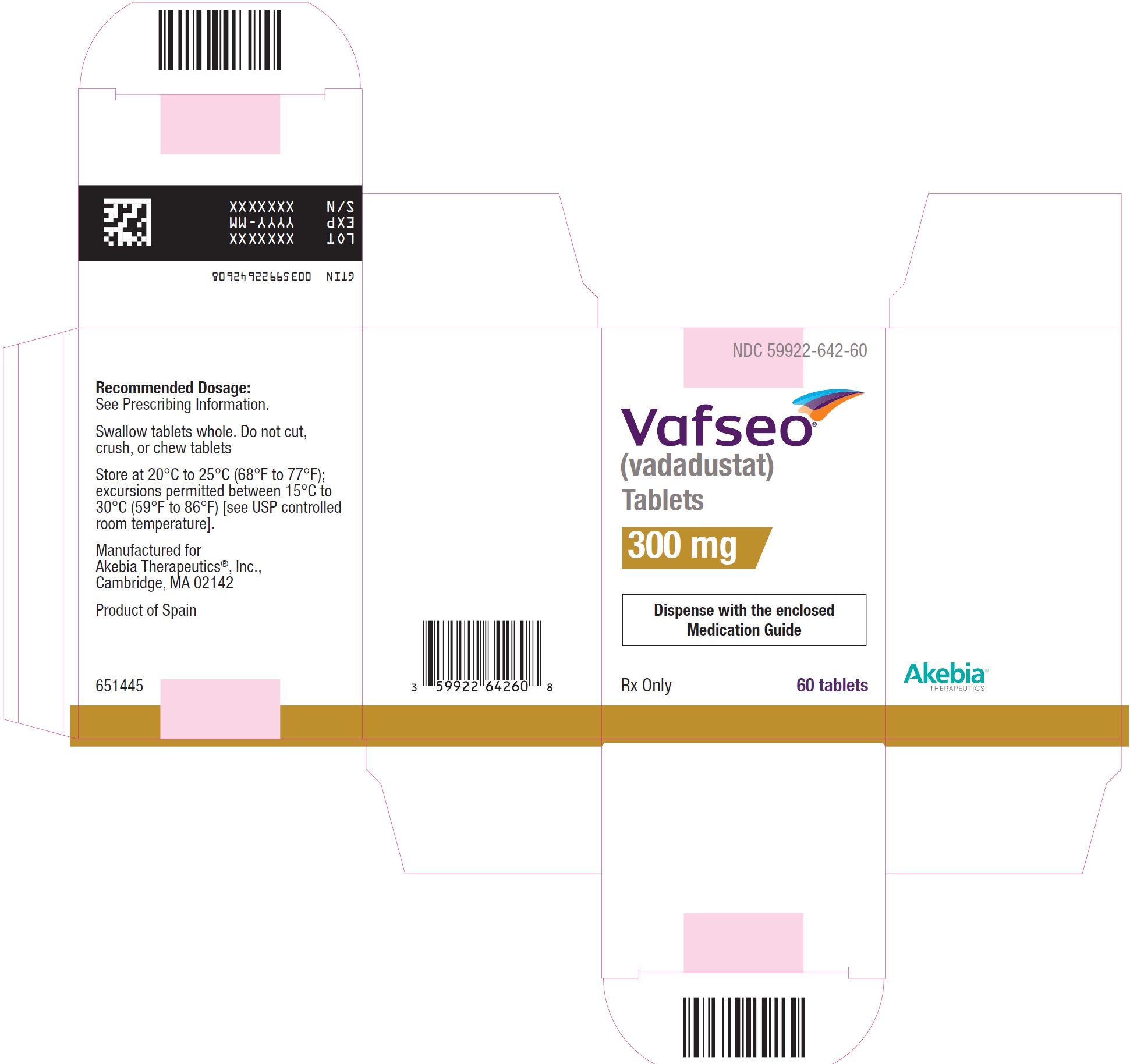
- PRINCIPAL DISPLAY PANEL - NDC: 59922-642-60 - 300 mg Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 59922-642-60 - 300 mg Bottle Label
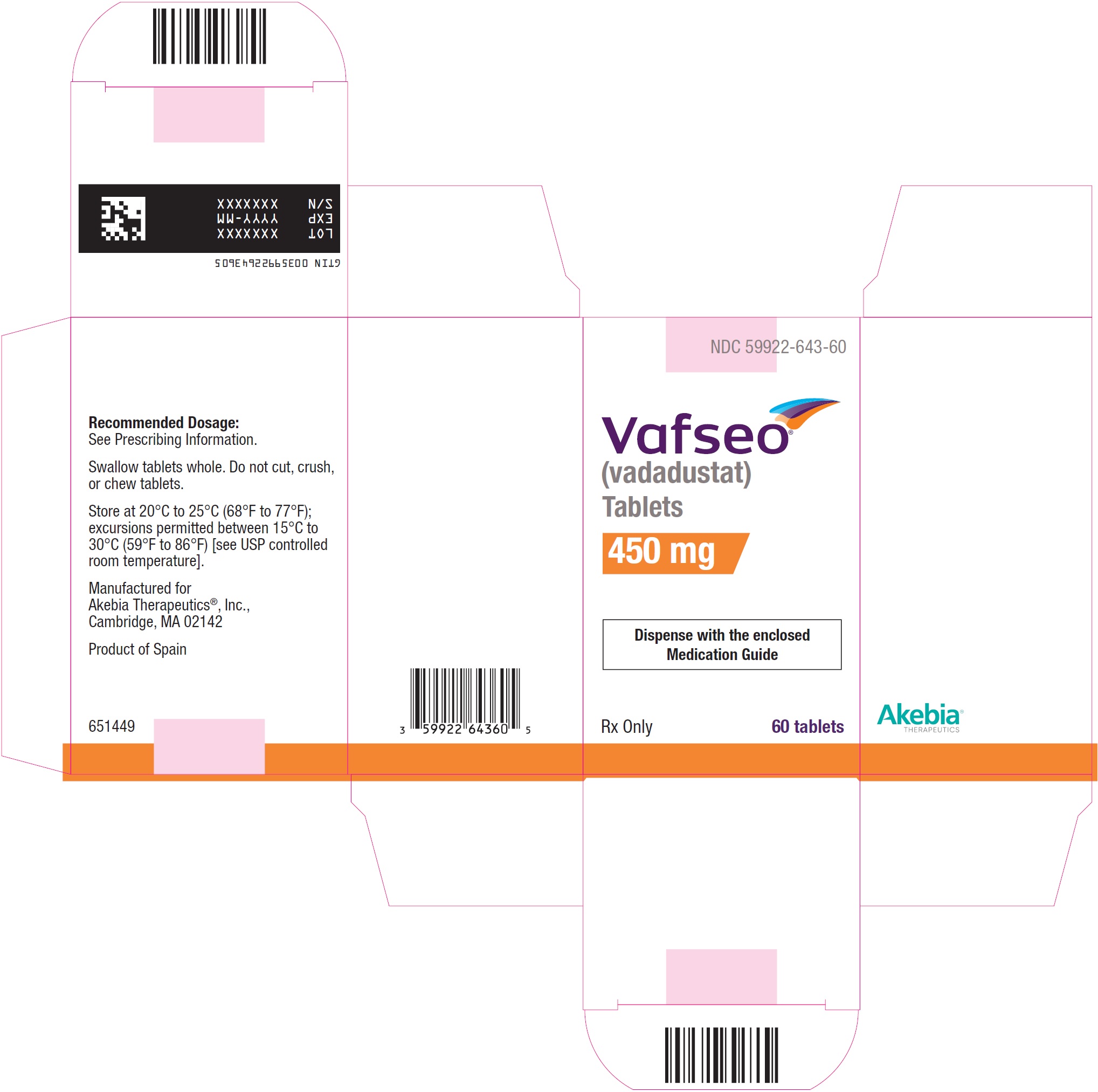
- PRINCIPAL DISPLAY PANEL - NDC: 59922-643-60 - 450 mg Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 59922-643-60 - 450 mg Bottle Label
-
INGREDIENTS AND APPEARANCE
VAFSEO
vadadustat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59922-641 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VADADUSTAT (UNII: I60W9520VV) (VADADUSTAT - UNII:I60W9520VV) VADADUSTAT 150 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code 150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59922-641-60 1 in 1 CARTON 03/28/2024 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215192 03/28/2024 VAFSEO
vadadustat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59922-642 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VADADUSTAT (UNII: I60W9520VV) (VADADUSTAT - UNII:I60W9520VV) VADADUSTAT 300 mg Product Characteristics Color YELLOW Score no score Shape OVAL Size 13mm Flavor Imprint Code 300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59922-642-60 1 in 1 CARTON 03/28/2024 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215192 03/28/2024 VAFSEO
vadadustat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59922-643 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VADADUSTAT (UNII: I60W9520VV) (VADADUSTAT - UNII:I60W9520VV) VADADUSTAT 450 mg Product Characteristics Color PINK Score no score Shape OVAL Size 15mm Flavor Imprint Code 450 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59922-643-60 1 in 1 CARTON 03/28/2024 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215192 03/28/2024 Labeler - Akebia Therapeutics, Inc. (809557593) Establishment Name Address ID/FEI Business Operations Esteve Quimica, S.A. 633485529 API MANUFACTURE(59922-641, 59922-642, 59922-643) , ANALYSIS(59922-641, 59922-642, 59922-643) Establishment Name Address ID/FEI Business Operations Changzhou SynTheAll Pharmaceutical Co., Ltd) 544385021 API MANUFACTURE(59922-641, 59922-642, 59922-643) , ANALYSIS(59922-641, 59922-642, 59922-643) Establishment Name Address ID/FEI Business Operations Patheon Inc. 205475333 MANUFACTURE(59922-641, 59922-642, 59922-643) , ANALYSIS(59922-641, 59922-642, 59922-643) Establishment Name Address ID/FEI Business Operations Shanghai STA Pharmaceutical Product Co., Ltd. 544561353 MANUFACTURE(59922-641, 59922-642, 59922-643) , ANALYSIS(59922-641, 59922-642, 59922-643)