Label: IMLYGIC- talimogene laherparepvec injection, suspension
- NDC Code(s): 55513-078-01, 55513-079-01
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IMLYGIC safely and effectively. See full prescribing information for IMLYGIC. IMLYGIC® (talimogene ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after ...
-
2 DOSAGE AND ADMINISTRATION
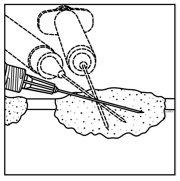
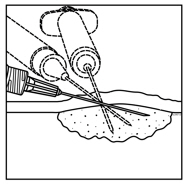
For intralesional injection only. Do not administer intravenously. 2.1 Dose - Administer IMLYGIC by injection into cutaneous, subcutaneous, and/or nodal lesions that are visible ...
-
3 DOSAGE FORMS AND STRENGTHS
Initial dose only: 106 (1 million) PFU per mL solution in 1 mL single-use vial (light green cap) Subsequent doses: 108 (100 million) PFU per mL solution in 1 mL single-use vial (royal blue ...
-
4 CONTRAINDICATIONS
4.1 Immunocompromised Patients - IMLYGIC is a live, attenuated herpes simplex virus and may cause life-threatening disseminated herpetic infection in patients who are immunocompromised ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure to IMLYGIC - Accidental exposure may lead to transmission of IMLYGIC and herpetic infection. Accidental needle stick and splashback to the eyes have been reported ...
-
6 ADVERSE REACTIONS
The most commonly reported adverse drug reactions (≥ 25%) in IMLYGIC-treated patients were fatigue, chills, pyrexia, nausea, influenza-like illness, and injection site pain. The following adverse ...
-
7 DRUG INTERACTIONS
IMLYGIC is sensitive to acyclovir. Acyclovir or other antiherpetic viral agents may interfere with the effectiveness of IMLYGIC. No drug interaction studies have been conducted with IMLYGIC.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Adequate and well-controlled studies with IMLYGIC have not been conducted in pregnant women. No effects on embryo-fetal development have been observed in a ...
-
10 OVERDOSAGE
There is no clinical experience with an overdose with IMLYGIC. Doses up to 4 mL at dose strength of 108 (100 million) PFU per mL every 2 weeks (maximum cumulative dose of 222.5 x 108 PFU) have ...
-
11 DESCRIPTION
IMLYGIC (talimogene laherparepvec) is a sterile suspension for intralesional injection. IMLYGIC is a live, attenuated HSV-1 that has been genetically modified to express huGM-CSF. The parental ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - IMLYGIC has been genetically modified to replicate within tumors and to produce the immune stimulatory protein GM-CSF. IMLYGIC causes lysis of tumors ...
-
13 NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology - Repeated intratumoral administration at 2 x 108 (200 million) PFU per kg (30-fold maximum proposed clinical dose, extrapolated based on body ...
-
14 CLINICAL STUDIES
The safety and efficacy of intralesional injections of IMLYGIC compared with subcutaneously administered GM-CSF was evaluated in a multicenter, open-label, randomized clinical study in patients ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - IMLYGIC is provided as a sterile frozen suspension in a single-use, cyclic olefin polymer (COP) plastic resin vial with a chlorobutyl elastomer stopper, aluminum seal, and ...
-
17 PATIENT COUNSELING INFORMATION
Advise patients and/or close contacts to: Read the FDA-approved patient labeling (Medication Guide). Follow instructions below to prevent viral transmission [see Warnings and Precautions ...
-
Medication Guide
IMLYGIC® (imm-LY-jik) (talimogene laherparepvec) Read the Medication Guide before you start treatment with IMLYGIC and before each IMLYGIC treatment. There may be new information. This ...
-
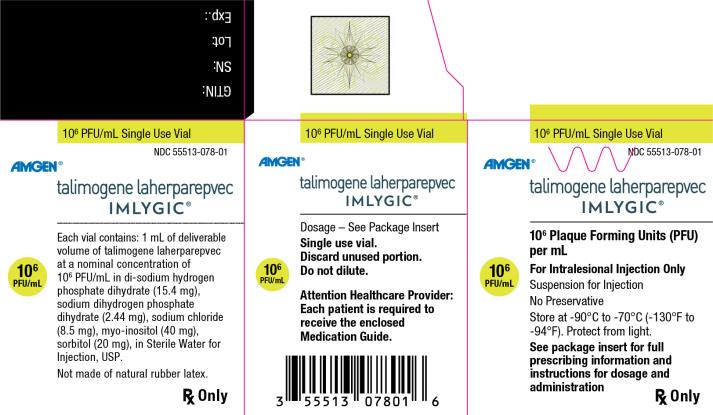
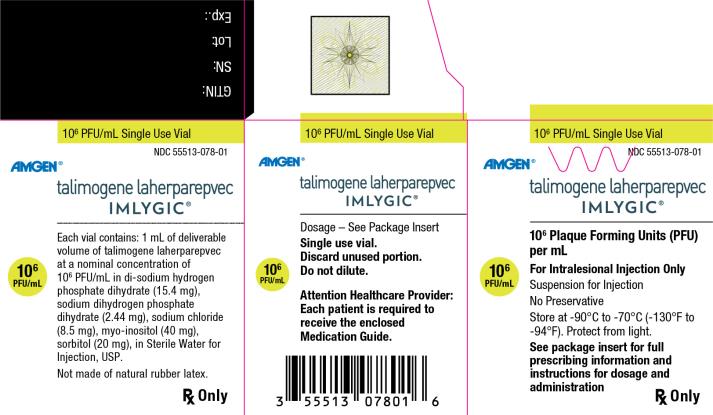
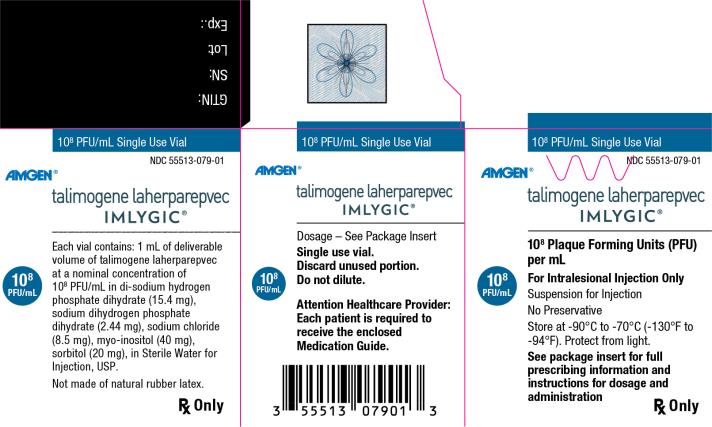
Principal Display Panel
106 PFU/mL Single Use Vial - NDC 55513-078-01 - Amgen® talimogene laherparepvec - IMLYGIC® 106 Plaque Forming Units (PFU) per mL - 106 PFU/mL - For Intralesional Injection Only - Suspension for Injection - No ...
-
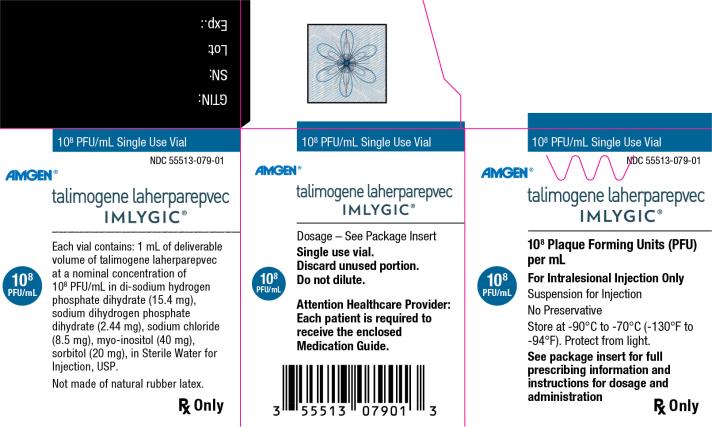
Principal Display Panel
108 PFU/mL Single Use Vial - NDC 55513-079-01 - Amgen® talimogene laherparepvec - IMLYGIC® 108 Plaque Forming Units (PFU) per mL - 108 PFU/mL - For Intralesional Injection Only - Suspension for Injection - No ...
-
INGREDIENTS AND APPEARANCEProduct Information