Label: REVUFORJ- revumenib tablet, film coated
- NDC Code(s): 73555-500-00, 73555-501-00, 73555-502-00
- Packager: Syndax Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REVUFORJ safely and effectively. See full prescribing information for REVUFORJ. Revuforj (revumenib) tablets for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME
Differentiation syndrome, which can be fatal, has occurred with REVUFORJ. Signs and symptoms may include fever, dyspnea, hypoxia, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, hypotension, and renal dysfunction. If differentiation syndrome is suspected, immediately initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution. [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Close -
1 INDICATIONS AND USAGERelapsed or Refractory Acute Leukemia - REVUFORJ is indicated for the treatment of relapsed or refractory acute leukemia with a lysine methyltransferase 2A gene (KMT2A) translocation in adult ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients for the treatment of acute leukemia with REVUFORJ based on the presence of a KMT2A translocation in bone marrow cells [see Clinical Studies (14.1)]. An ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 25 mg of revumenib: Pink modified oval film-coated tablet debossed with “S” on one side and “25” on the other side. 110 mg of revumenib: Beige modified oval film-coated tablet ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Differentiation Syndrome - REVUFORJ can cause fatal or life-threatening differentiation syndrome (DS). Symptoms of differentiation syndrome, including those seen in patients treated with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Differentiation Syndrome [see Warnings and Precautions (5.1)] QTc Interval Prolongation [see ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on REVUFORJ - Strong CYP3A4 Inhibitors - If concomitant use of strong CYP3A4 inhibitors is required, reduce the REVUFORJ dosage [see Recommended Dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals and its mechanism of action [see Clinical Pharmacology (12.1)], REVUFORJ can cause fetal harm when administered to a pregnant ...
-
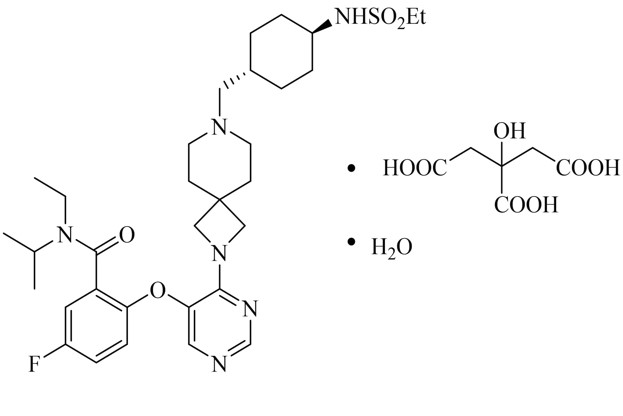
11 DESCRIPTIONREVUFORJ contains revumenib, a menin inhibitor. Revumenib is present as revumenib citrate hydrate with a chemical name of benzamide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Revumenib is a menin inhibitor and blocks the interaction of both wild-type lysine methyltransferase 2A (KMT2A) and KMT2A fusion proteins with menin. The binding of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with revumenib. In a repeat dose toxicity study in rats treated with revumenib for 13 ...
-
14 CLINICAL STUDIES14.1 Relapsed or Refractory Acute Leukemia with a KMT2A Translocation - SNDX-5613-0700 - The efficacy of REVUFORJ was evaluated in a single-arm cohort of an open-label, multicenter trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING25 mg: Pink modified oval film-coated tablet debossed with “S” on one side and “25” on the other side. 30-count bottles with a desiccant and child resistant closure (NDC 73555-500-00) 110 mg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) and Instructions for Use. Differentiation Syndrome - Advise patients of the risks of developing differentiation ...
-
SPL UNCLASSIFIED SECTIONManufactured for - Syndax Pharmaceuticals, Inc., Waltham, MA 02451 - REVUFORJ ® is a registered trademark of Syndax Pharmaceuticals, Inc. © 2024 Syndax Pharmaceuticals ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug AdministrationIssued: 11/2024 - Medication Guide REVUFORJ (REV-you-forge) (revumenib) tablets, for oral use - What is ...
-
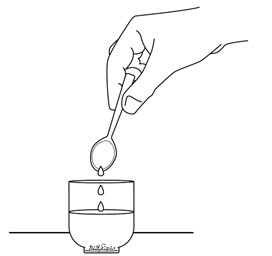
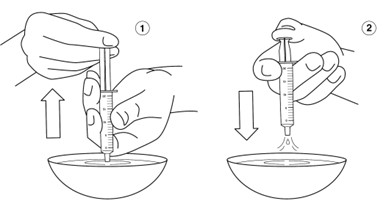
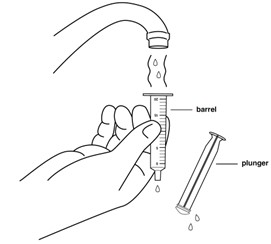
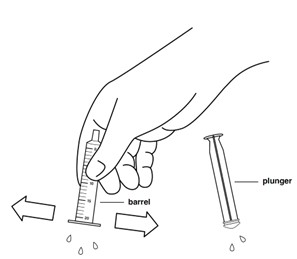
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - REVUFORJ (REV-you-forge) revumenib - tablets, for oral use - Read these Instructions for Use to prepare and take or give a dose of REVUFORJ tablets broken apart ...
-
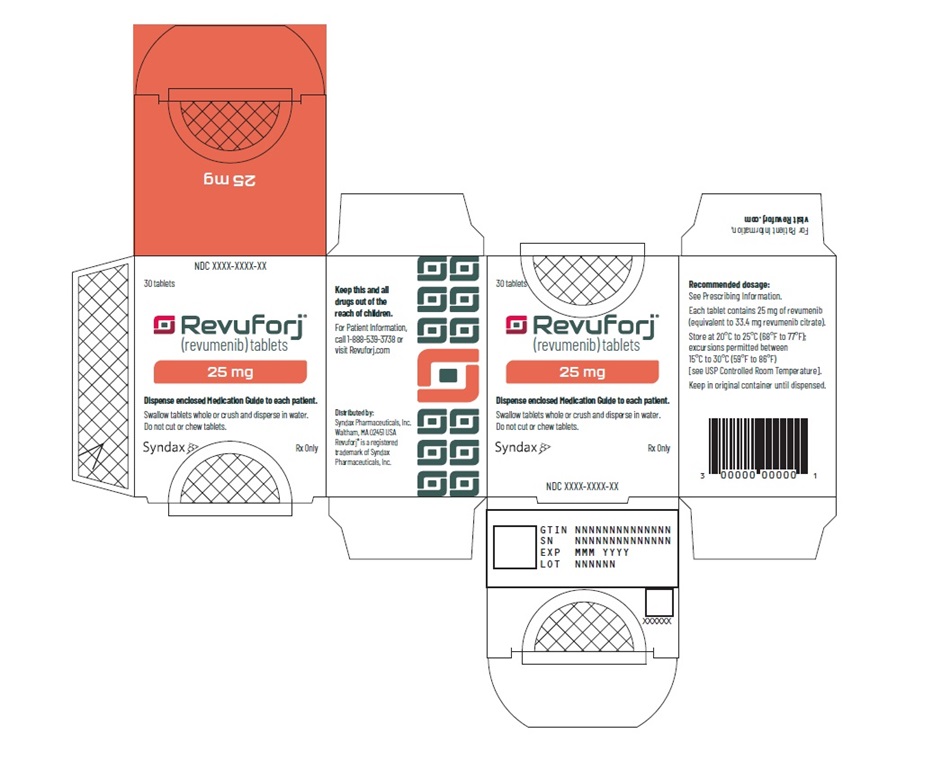
Revuforj 25 mg carton/container labelsPrinciple Display Panel - 30 tablets - Revuforj® (revumenib) tablets - 25 mg - Dispense enclosed Medication Guide to each patient. Swallow tablets whole or crush and disperse in water. Do not cut ...
-
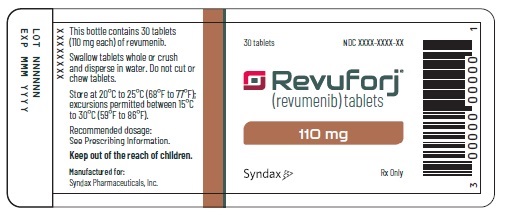
Revuforj 110 mg carton/container labelsPrinciple Display Panel - 30 tablets - Revuforj® (revumenib) tablets - 110 mg - Dispense enclosed Medication Guide - to each patient. Swallow tablets whole or crush and - disperse in water. Do not ...
-
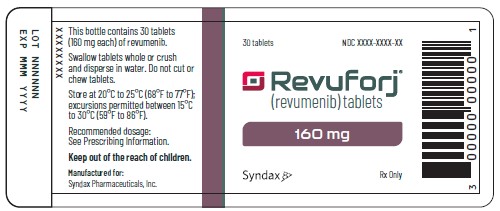
Revuforj 160 mg carton/container labelsPrinciple Display Panel - 30 tablets - Revuforj® (revumenib) tablets - 160 mg - Dispense enclosed Medication Guide - to each patient. Swallow tablets whole or crush and - disperse in water. Do not ...
-
INGREDIENTS AND APPEARANCEProduct Information