Label: RIVIVE- naloxone hydrochloride spray
- NDC Code(s): 82954-0100-1
- Packager: Harm Reduction Therapeutics, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
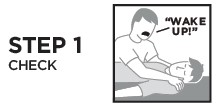
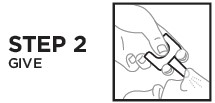
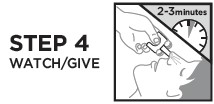
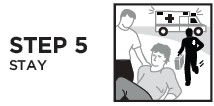
Directions
- Warning
- Other information
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RIVIVE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82954-0100 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 3 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82954-0100-1 2 in 1 CARTON 01/02/2024 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217722 01/02/2024 Labeler - Harm Reduction Therapeutics, Inc. (107812954)