Label: QLOSI- pilocarpine hydrochloride solution
- NDC Code(s): 83661-018-10, 83661-018-20, 83661-018-30, 83661-018-60
- Packager: Orasis Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
QLOSI
These highlights do not include all the information needed to use see full prescribing information for Initial U.S. ApprovalINDICATIONS AND USAGE
QLOSI is a cholinergic agonist indicated for the treatment of presbyopia in adults. ( 1)
DOSAGE AND ADMINISTRATION
Instill one drop of QLOSI in each eye. This can be repeated a second time after 2 to 3 hours for an effect up to 8 hours. QLOSI can be administered on a daily basis, or as needed, up to twice each day. ( 2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: pilocarpine hydrochloride 0.4% (4 mg/mL) in a single-patient-use vial. ( 3)
CONTRAINDICATIONS
Hypersensitivity ( 4)
WARNINGS AND PRECAUTIONS
Blurred Vision: Advise patients to not drive or operate machinery if vision is not clear (e.g., blurred vision). Exercise caution in night driving and other hazardous occupations in poor illumination. ( 5.1)
Risk of Retinal Detachment: Rare cases of retinal detachment have been reported with miotics. Examination of the retina is advised in all patients prior to initiation of therapy. Advise patients to seek immediate medical care with sudden onset of flashes of lights, floaters, or vision loss. ( 5.2)
Iritis: Caution is advised in patients with iritis. ( 5.3)
ADVERSE REACTIONS
Most common adverse reactions (5% to 8%) are instillation site pain and headaches. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Orasis Pharmaceuticals Inc. at 1-866-ORASIS1 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Blurred Vision
5.2 Risk of Retinal Detachment
5.3 Iritis
5.4 Contact Lens Wear
5.5 Potential for Eye Injury or Contamination
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
17 PATIENT COUNSELING INFORMATION
16.1 How Supplied
16.2 Storage
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Instill one drop of QLOSI in each eye. This can be repeated a second time after 2 to 3 hours for an effect up to 8 hours. QLOSI can be administered on a daily basis, or as needed, up to twice each day.
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
Discard single-patient-use vial after use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Blurred Vision
Miotics, including QLOSI, may cause accommodative spasm. Advise patients to not drive or operate machinery if vision is not clear (e.g., blurred vision).
In addition, patients may experience temporary dim or dark vision with miotics, including QLOSI. Advise patients to exercise caution in night driving and other hazardous activities in poor illumination.
5.2 Risk of Retinal Detachment
Rare cases of retinal detachment have been reported with miotics when used in susceptible individuals and those with pre-existing retinal disease. Examination of the retina is advised in all patients prior to the initiation of therapy. Advise patients to seek immediate medical care with sudden onset of flashes of lights, floaters, or vision loss.
5.3 Iritis
QLOSI is not recommended to be used when iritis is present because adhesions (synechiae) may form between the iris and the lens.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
QLOSI was evaluated in 309 patients with presbyopia in two randomized, double-masked, vehicle-controlled studies (NEAR-1 and NEAR-2) of 15 days duration. The most commonly reported treatment-related adverse events in 5-8% of patients were instillation site pain, and headache. Ocular adverse reaction reported in 2-5% of patients was blurred vision. The majority of the adverse events were mild, transient and self-resolving.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of QLOSI administration in pregnant women to inform a drug associated risk. Oral administration of pilocarpine to pregnant rats throughout organogenesis and lactation did not produce adverse effects at clinically relevant doses.
Data
Animal Data
In embryofetal development studies, oral administration of pilocarpine to pregnant rats throughout organogenesis produced maternal toxicity, skeletal anomalies and reduction in fetal body weight at 90 mg/kg/day (approximately 1200-fold higher than the maximum recommended human ophthalmic dose [MRHOD] of 0.012mg/kg/day, on a mg/m 2basis).
In peri-/postnatal study in rats, oral administration of pilocarpine during late gestation through lactation increased stillbirths at a dose of 36 mg/kg/day (approximately 475-fold higher than the MRHOD of 0.012mg/kg/day, on a mg/m 2basis). Decreased neonatal survival and reduced mean body weight of pups were observed at ≥18mg/kg/day (approximately 240-fold higher than the MRHOD of 0.012mg/kg/day, on a mg/m 2basis).
8.2 Lactation
Risk Summary
There are no data on the presence of pilocarpine in human milk, the effects on breastfed infants, or the effects on milk production to inform the risk of QLOSI to an infant during lactation. Pilocarpine and/or its metabolites are excreted in the milk of lactating rats following single oral administration. Systemic levels of pilocarpine following topical ocular administration are low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of pilocarpine would be present in maternal milk following ocular administration of QLOSI.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for QLOSI and any potential adverse effects on the breastfed child from QLOSI.
8.4 Pediatric Use
Presbyopia is an age-related condition which does not appear in the pediatric population.
8.5 Geriatric Use
Clinical studies of QLOSI did not include subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with ophthalmic pilocarpine solutions have not identified overall differences in safety between elderly and younger patients. Safety and effectiveness have not been established in patients over the age of 65.
-
10 OVERDOSAGE
Systemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity.
Accidental ingestion can produce sweating, salivation, nausea, tremors and slowing of the pulse and a decrease in blood pressure.
In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration. For patients demonstrating severe poisoning, atropine, the pharmacologic antagonist to pilocarpine, should be used.
-
11 DESCRIPTION
QLOSI (pilocarpine hydrochloride ophthalmic solution) 0.4% is a sterile, clear, colorless to slightly yellowish and slightly viscous ophthalmic solution for topical ophthalmic use which has a pH between 5.1-6.1.
The structural formula of pilocarpine hydrochloride is:
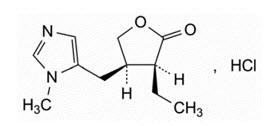
The chemical name for pilocarpine hydrochloride is: 2(3 H)-Furanone, 3-ethyldihydro-4-[(1-methyl-1 H-imidazol-5-yl)- methyl]-monohydrochloride, (3 S-cis). Its molecular weight is 244.72 g/mol and its molecular formula is C 11H 16N 2O 2∙ HCl.
Each mL contains 0.4% (4 mg) of pilocarpine hydrochloride as the active ingredient, equivalent to 0.3% (3.4 mg) of pilocarpine free-base. The inactive ingredients in QLOSI are: hypromellose, sodium chloride, sodium hyaluronate, edetate disodium dihydrate, water for injection, and may also include sodium hydroxide and/or hydrochloric acid for pH adjustment. QLOSI does not contain an anti-microbial preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pilocarpine hydrochloride is a cholinergic muscarinic agonist which activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ciliary muscle. QLOSI contracts the iris sphincter muscle, constricting the pupil to enhance the depth of focus and improve near visual acuity while maintaining some pupillary response to light.
12.3 Pharmacokinetics
Systemic absorption of pilocarpine was evaluated in 12 healthy subjects following QLOSI administration (2 doses daily in each eye, 2 hours apart, for 8 days). The overall median T maxin plasma after Day 8 dosing was 2.20 hr and the mean (SD) overall plasma C maxand AUC 0-tafter Day 8 dosing were 897.2 (287.2) pg/mL and 2699 (741.4) hr*pg/mL, respectively. The mean T 1/2after Day 8 dosing was 3.96 hr.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime oral carcinogenicity studies were conducted in CD-1 mice and Sprague-Dawley rats. Pilocarpine did not induce tumors in mice at any dosage level studies (up to 30 mg/kg/day; approximately 200-fold higher than the MRHOD of 0.012mg/kg/day, on a mg/m 2basis). In rats, an oral dose of 18 mg/kg/day (approximately 240-fold higher than the MRHOD), resulted in a statistically significant increase in the incidence of benign pheochromocytomas in both male and female rats, and a statistically significant increase in the incidence of hepatocellular adenomas in female rats.
Mutagenesis
Pilocarpine did not show any potential to cause genetic toxicity in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in a Chinese hamster ovary cell line; 3) an in vivo chromosome aberration assay (micronucleus test) in mice; and 4) a primary DNA damage assay (unscheduled DNA synthesis) in rat hepatocyte primary cultures.
Impairment of Fertility
Pilocarpine oral administration to male and female rats at a dosage of 18 mg/kg/day (approximately 240-fold higher than the MRHOD of 0.012mg/kg/day, on a mg/m 2basis) resulted in impaired reproductive function, including reduced fertility, decreased sperm motility, and morphological evidence of abnormal sperm. It is unclear whether the reduction in fertility was due to effects on males, females, or both. In dogs, exposure to pilocarpine at a dosage of 3mg/kg/day for 6 months resulted in evidence of impaired spermatogenesis (approximately 135-fold higher than the MHROD of 0.012mg/kg/day, on a mg/m 2basis).
-
14 CLINICAL STUDIES
The efficacy of QLOSI for the treatment of presbyopia was demonstrated in two Phase 3, randomized, double-masked, vehicle-controlled studies, namely NEAR-1 (NCT04599933) and NEAR-2 (NCT04599972). A total of 613 participants aged 45 to 64 years old with presbyopia were randomized (309 to QLOSI group) in these two studies. Participants were instructed to instill one drop of QLOSI or vehicle, in each eye, once in the morning and to repeat the instillation 2 to 3 hours later. Participants were treated for two weeks. Ophthalmic assessments were conducted on Day 1, 8 and 15 of the study at various timepoints.
Responders demonstrated improvement by achieving a gain from baseline of 3 lines or more in near BDCVA at 40 centimeters without a loss of 1 line or more (≥ 5 letters) in BDCVA at 4 meters. Overall, the percentages of responders were consistently higher in the QLOSI group than in the Vehicle group at each assessment day.
Table 1: Primary and key secondary results from NEAR-1 and NEAR-2 studies: Percentage of Responders - Day 8 NEAR-1 NEAR-2 Assessment timing QLOSI N=155 Vehicle N=154 p-value QLOSI N=154 Vehicle N=150 p-value Abbreviations: N: number of participants in this study arm 1 hour post Dose 1 39% 17% <0.01 42% 21% <0.01 2 hours post Dose 1 39% 17% <0.01 40% 21% <0.01 1 hour post Dose 2 48% 16% <0.01 52% 17% <0.01 2 hours post Dose 2 39% 15% <0.01 46% 19% <0.01 Figure 1 presents the percentage of responders for each time point at Day 15. (Standard analysis of pooled data NEAR-1 and NEAR-2)
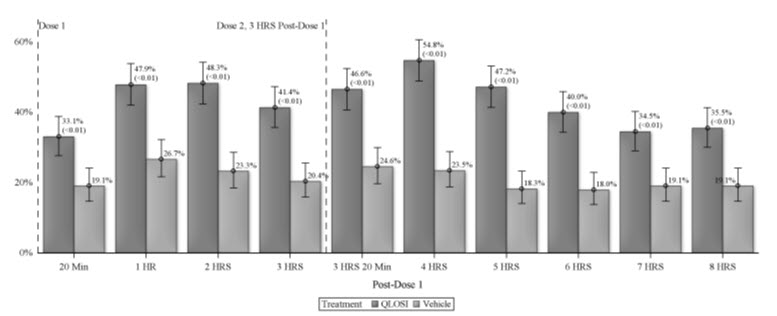
Abbreviations: HRS: hours; min: minutes
This figure demonstrates the onset of the QLOSI effect on presbyopia, from 20 minutes post dose 1, and lasting up to 8 hours (i.e., 5 hours from the second dose).
-
17 PATIENT COUNSELING INFORMATION
Night Driving
QLOSI may cause temporary dim or dark vision. Advise patients to exercise caution with night driving and when hazardous activities are undertaken in poor illumination.
Blurred Vision
Temporary problems when changing focus between near objects and distant objects may occur. Advise patients to not drive or use machinery if vision is not clear.
When to Seek Physician Advice
Advise patients to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss [see Warnings and Precautions (5.2)].
Contact Lens Wear
Advise patients to remove contact lenses prior to the instillation of QLOSI. Wait 10 minutes after dosing before reinserting contact lenses.
Potential for Eye Injury or Contamination
Advise patients to avoid touching the tip of the single-patient-use vial to the eye or to any other surface, in order to prevent eye injury or contamination.
-
16 HOW SUPPLIED/ STORAGE AND HANDLING
16.1 How Supplied
QLOSI is supplied as a sterile, clear, colorless to slightly yellowish and slightly viscous ophthalmic solution in configurations of 5 single-patient-use vials of 0.4 mL fill. Each single-patient-use vial is comprised of transparent low-density polyethylene (LDPE).
5 single-patient-use vials are packaged in a foil pouch.
QLOSI is supplied in:
NDC 83661-018-10: carton box of 10 single-patient-use vials (2 pouches containing 5 vials)
NDC 83661-018-20: carton box of 20 single-patient-use vials (4 pouches containing 5 vials)
NDC 83661-018-30: carton box of 30 single-patient-use vials (6 pouches containing 5 vials)
NDC 83661-018-60: carton box of 60 single-patient-use vials (12 pouches containing 5 vials)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.4 mL Vial Box
-
INGREDIENTS AND APPEARANCE
QLOSI
pilocarpine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83661-018 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PILOCARPINE HYDROCHLORIDE (UNII: 0WW6D218XJ) (PILOCARPINE - UNII:01MI4Q9DI3) PILOCARPINE HYDROCHLORIDE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYALURONATE SODIUM (UNII: YSE9PPT4TH) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83661-018-30 30 in 1 BOX 10/15/2024 1 0.4 mL in 1 VIAL, SINGLE-USE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:83661-018-10 10 in 1 BOX 01/15/2025 2 0.4 mL in 1 VIAL, SINGLE-USE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:83661-018-20 20 in 1 BOX 01/15/2025 3 0.4 mL in 1 VIAL, SINGLE-USE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:83661-018-60 60 in 1 BOX 01/15/2025 4 0.4 mL in 1 VIAL, SINGLE-USE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217836 10/15/2024 Labeler - Orasis Pharmaceuticals, Inc. (118766011)


