Label: POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
- NDC Code(s): 50242-103-01, 50242-105-01
- Packager: Genentech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POLIVY safely and effectively. See full prescribing information for POLIVY. POLIVY® (polatuzumab vedotin-piiq) for injection, for ...These highlights do not include all the information needed to use POLIVY safely and effectively. See full prescribing information for POLIVY.
POLIVY® (polatuzumab vedotin-piiq) for injection, for intravenous use
Initial U.S. Approval: 2019RECENT MAJOR CHANGES
INDICATIONS AND USAGE
POLIVY is a CD79b-directed antibody and microtubule inhibitor conjugate indicated:
- in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for the treatment of adult patients who have previously untreated diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS) or high-grade B-cell lymphoma (HGBL) and who have an International Prognostic Index score of 2 or greater. (1.1)
- in combination with bendamustine and a rituximab product for the treatment of adult patients with relapsed or refractory DLBCL, NOS, after at least two prior therapies. (1.2)
DOSAGE AND ADMINISTRATION
- The recommended dose of POLIVY is 1.8 mg/kg as an intravenous infusion every 21 days for 6 cycles. (2)
- Administer the initial POLIVY dose over 90 minutes. Subsequent infusions may be administered over 30 minutes if the previous infusion is tolerated. (2)
- Premedicate with an antihistamine and antipyretic before POLIVY. (2)
- See Full Prescribing Information for instructions on preparation and administration. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 30 mg or 140 mg of polatuzumab vedotin-piiq as a lyophilized powder in a single-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Peripheral Neuropathy: Monitor patients for peripheral neuropathy and modify or discontinue dose accordingly. (5.1)
- Infusion-Related Reactions: Premedicate with an antihistamine and antipyretic. Monitor patients closely during infusions. Interrupt or discontinue infusion for reactions. (5.2)
- Myelosuppression: Monitor complete blood counts. Manage using dose delays or reductions and growth factor support. Monitor for signs of infection. (5.3)
- Serious and Opportunistic Infections: Closely monitor patients for signs of bacterial, fungal, or viral infections. (5.4)
- Progressive Multifocal Leukoencephalopathy (PML): Monitor patients for new or worsening neurological, cognitive, or behavioral changes suggestive of PML. (5.5)
- Tumor Lysis Syndrome: Closely monitor patients with high tumor burden or rapidly proliferative tumors. (5.6)
- Hepatotoxicity: Monitor liver enzymes and bilirubin. (5.7)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment and for 3 months after the last dose. (5.8)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) in patients with large B-cell lymphoma treated with POLIVY in combination with R-CHP, excluding laboratory abnormalities, are peripheral neuropathy, nausea, fatigue, diarrhea, constipation, alopecia, and mucositis. Grade 3 to 4 laboratory abnormalities (≥10%) are lymphopenia, neutropenia, hyperuricemia, and anemia. (6.1)
The most common adverse reaction (≥20%) in patients with relapsed or refractory DLBCL treated with POLIVY in combination with BR are neutropenia, thrombocytopenia, anemia, peripheral neuropathy, fatigue, diarrhea, pyrexia, decreased appetite, and pneumonia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant use of strong CYP3A inhibitors or inducers has the potential to affect the exposure to unconjugated monomethyl auristatin E (MMAE). (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Previously Untreated DLBCL, NOS or HGBL
1.2 Relapsed or Refractory DLBCL, NOS
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Management of Adverse Reactions
2.3 Recommended Prophylactic Medications
2.4 Instructions for Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Peripheral Neuropathy
5.2 Infusion-Related Reactions
5.3 Myelosuppression
5.4 Serious and Opportunistic Infections
5.5 Progressive Multifocal Leukoencephalopathy (PML)
5.6 Tumor Lysis Syndrome
5.7 Hepatotoxicity
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on POLIVY
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Previously Untreated DLBCL, NOS or HGBL
14.2 Relapsed or Refractory DLBCL, NOS
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Previously Untreated DLBCL, NOS or HGBL - POLIVY in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone (R-CHP) is indicated for the treatment of adult ...
1.1 Previously Untreated DLBCL, NOS or HGBL
POLIVY in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone (R-CHP) is indicated for the treatment of adult patients who have previously untreated diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS) or high-grade B-cell lymphoma (HGBL) and who have an International Prognostic Index score of 2 or greater.
Close1.2 Relapsed or Refractory DLBCL, NOS
POLIVY in combination with bendamustine and a rituximab product is indicated for the treatment of adult patients with relapsed or refractory DLBCL, NOS, after at least two prior therapies.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Patients with Previously Untreated DLBCL, NOS or HGBL - The recommended dosage of POLIVY is 1.8 mg/kg administered as an intravenous infusion every 21 days for 6 ...
2.1 Recommended Dosage
Patients with Previously Untreated DLBCL, NOS or HGBL
The recommended dosage of POLIVY is 1.8 mg/kg administered as an intravenous infusion every 21 days for 6 cycles in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone [see Clinical Studies (14.1)]. Administer POLIVY, cyclophosphamide, doxorubicin, and a rituximab product in any order on Day 1 after the administration of prednisone. Prednisone is administered on Days 1–5 of each cycle.
Patients with Relapsed or Refractory DLBCL, NOS
The recommended dosage of POLIVY is 1.8 mg/kg administered as an intravenous infusion every 21 days for 6 cycles in combination with bendamustine and a rituximab product. Administer POLIVY, bendamustine, and a rituximab product in any order on Day 1 of each cycle. The recommended dose of bendamustine is 90 mg/m2/day on Days 1 and 2 when administered with POLIVY and a rituximab product. The recommended dose of rituximab product is 375 mg/m2 intravenously on Day 1 of each cycle.
2.2 Management of Adverse Reactions
Previously Untreated DLBCL, NOS or HGBL
Table 1 provides management guidelines for peripheral neuropathy in patients receiving POLIVY plus R-CHP [see Warnings and Precautions (5.1)].
Table 1 Management of Peripheral Neuropathy in Patients Receiving POLIVY Plus R-CHP Adverse reaction Grade Dose modification* R-CHP should be continued if POLIVY is withheld.
If there is concurrent sensory and motor neuropathy, follow the guidance for the most severe neuropathy. If the grade of sensory and motor neuropathy are the same, follow the guidance for motor neuropathy.- *
- Starting dose for POLIVY is 1.8 mg/kg. First dose reduction level is 1.4 mg/kg. Second dose reduction level is 1 mg/kg. No further dose reduction is recommended beyond 1 mg/kg. If further reduction needed discontinue POLIVY.
Peripheral sensory neuropathy Grade 1 None Grade 2 If resolves to Grade 1 or lower before the next scheduled dose, resume at the same dose level.
If Gr 2 persists at the next scheduled dose, reduce one dose level.Grade 3 Withhold until Grade 2 or lower and reduce one dose level. Grade 4 Permanently discontinue. Peripheral motor neuropathy Grade 1 None Grade 2 or 3 Withhold until Grade 1 or lower and reduce one dose level. Grade 4 Permanently discontinue. Table 2 provides management guidelines for infusion-related reaction and myelosuppression [see Warnings and Precautions (5.2 and 5.3)].
Table 2 Management of Infusion-Related Reaction and Myelosuppression in Patients Receiving POLIVY Plus R-CHP Adverse Reaction Dosage Modification* Toxicity graded per National Cancer Institute (NCI) Common Terminology for Adverse Events (CTCAE) version 4.0. - *
- Starting dose for POLIVY is 1.8 mg/kg. First dose reduction level is 1.4 mg/kg. Second dose reduction level is 1 mg/kg. No further dose reduction is recommended beyond 1 mg/kg. If further reduction needed discontinue POLIVY.
- †
- Severity on Day 1 of any cycle.
- ‡
- If primary cause is lymphoma, dosage delay or reduction may not be needed.
Infusion-Related Reaction
Grade 1–3Interrupt POLIVY infusion and give supportive treatment.
For the first instance of Grade 3 wheezing, bronchospasm, or generalized urticaria, permanently discontinue POLIVY.
For recurrent Grade 2 wheezing or urticaria, or for recurrence of any Grade 3 symptoms, permanently discontinue POLIVY.
Otherwise, upon complete resolution of symptoms, infusion may be resumed at 50% of the rate achieved prior to interruption. In the absence of infusion related symptoms, the rate of infusion may be escalated in increments of 50 mg/hour every 30 minutes.
For the next cycle, infuse POLIVY over 90 minutes. If no infusion-related reaction occurs, subsequent infusions may be administered over 30 minutes. Administer premedication for all cycles.Infusion-Related Reaction
Grade 4Stop POLIVY infusion immediately.
Give supportive treatment.
Discontinue POLIVY.Neutropenia†,‡
Grade 3–4Hold all treatment until ANC recovers to greater than or equal to 1,000/microliter.
Consider therapeutic G-CSF if neutropenia occurs after prophylactic G-CSF.
If ANC recovers to greater than or equal to 1,000/microliter on or before Day 7, resume all treatment without any dose reductions.
If ANC recovers to greater than or equal to 1,000/microliter after Day 7:- resume all treatment
- administer prophylactic G-CSF in next cycle. If G-CSF was already given, consider a dose reduction of POLIVY.
Thrombocytopenia†,‡
Grade 3–4Hold all treatment until platelets recover to greater than or equal to 75,000/microliter.
If platelets recover to greater than or equal to 75,000/microliter on or before Day 7, resume all treatment without any dose reductions.
If platelets recover to greater than or equal to 75,000/microliter after Day 7:- resume all treatment and consider a dose reduction of POLIVY.
Relapsed or Refractory DLBCL, NOS
Table 3 provides management guidelines for peripheral neuropathy, infusion-related reaction, and myelosuppression in patients receiving POLIVY in combination with bendamustine and a rituximab product [see Warnings and Precautions (5.1, 5.2, 5.3)].
Table 3 Management of Peripheral Neuropathy, Infusion-Related Reaction, and Myelosuppression in Patients Receiving POLIVY Plus Bendamustine and a Rituximab Product Adverse Reaction Dosage Modification* Toxicity graded per NCI CTCAE version 4.0. - *
- Starting dose for POLIVY is 1.8 mg/kg. First dose reduction level is 1.4 mg/kg. Second dose reduction level is 1 mg/kg. No further dose reduction is recommended beyond 1 mg/kg. If further reduction needed discontinue POLIVY.
- †
- Severity on Day 1 of any cycle.
- ‡
- If primary cause is lymphoma, dosage delay or reduction may not be needed.
Peripheral Neuropathy
Grade 2–3Hold POLIVY dosing until improvement to Grade 1 or lower.
If recovered to Grade 1 or lower on or before Day 14, restart POLIVY with the next cycle at a permanently reduced dose of 1.4 mg/kg.
If a prior dose reduction to 1.4 mg/kg has occurred, discontinue POLIVY.
If not recovered to Grade 1 or lower on or before Day 14, discontinue POLIVY.Peripheral Neuropathy
Grade 4Discontinue POLIVY. Infusion-Related Reaction
Grade 1–3Interrupt POLIVY infusion and give supportive treatment.
For the first instance of Grade 3 wheezing, bronchospasm, or generalized urticaria, permanently discontinue POLIVY.
For recurrent Grade 2 wheezing or urticaria, or for recurrence of any Grade 3 symptoms, permanently discontinue POLIVY.
Otherwise, upon complete resolution of symptoms, infusion may be resumed at 50% of the rate achieved prior to interruption. In the absence of infusion related symptoms, the rate of infusion may be escalated in increments of 50 mg/hour every 30 minutes.
For the next cycle, infuse POLIVY over 90 minutes. If no infusion-related reaction occurs, subsequent infusions may be administered over 30 minutes. Administer premedication for all cycles.Infusion-Related Reaction
Grade 4Stop POLIVY infusion immediately.
Give supportive treatment.
Permanently discontinue POLIVY.Neutropenia†,‡
Grade 3–4Hold all treatment until ANC recovers to greater than 1,000/microliter.
If ANC recovers to greater than 1,000/microliter on or before Day 7, resume all treatment without any additional dose reductions. Consider granulocyte colony-stimulating factor prophylaxis for subsequent cycles, if not previously given.
If ANC recovers to greater than 1,000/microliter after Day 7:- restart all treatment. Consider granulocyte colony-stimulating factor prophylaxis for subsequent cycles, if not previously given. If prophylaxis was given, consider dose reduction of bendamustine.
- if dose reduction of bendamustine has already occurred, consider dose reduction of POLIVY to 1.4 mg/kg.
Thrombocytopenia†,‡
Grade 3–4Hold all treatment until platelets recover to greater than 75,000/microliter.
If platelets recover to greater than 75,000/microliter on or before Day 7, resume all treatment without any additional dose reductions.
If platelets recover to greater than 75,000/microliter after Day 7:- restart all treatment, with dose reduction of bendamustine.
- if dose reduction of bendamustine has already occurred, consider dose reduction of POLIVY to 1.4 mg/kg.
2.3 Recommended Prophylactic Medications
If not already premedicated for a rituximab product, administer an antihistamine and antipyretic at least 30 to 60 minutes prior to POLIVY for potential infusion-related reactions [see Warnings and Precautions (5.2)].
Administer prophylaxis for Pneumocystis jiroveci pneumonia and herpesvirus throughout treatment with POLIVY.
Administer prophylactic granulocyte colony-stimulating factor (G-CSF) for neutropenia in patients receiving POLIVY plus R-CHP. Consider prophylactic G-CSF administration for neutropenia in patients receiving POLIVY plus bendamustine and a rituximab product [see Warnings and Precautions (5.3)].
Administer tumor lysis syndrome prophylaxis for patients at increased risk of tumor lysis syndrome [see Warnings and Precautions (5.6)].
Close2.4 Instructions for Preparation and Administration
Reconstitute and further dilute POLIVY prior to intravenous infusion.
POLIVY is a hazardous drug. Follow applicable special handling and disposal procedures.1
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Reconstitution
- Reconstitute immediately before dilution.
- More than one vial may be needed for a full dose. Calculate the dose, the total volume of reconstituted POLIVY solution required, and the number of POLIVY vials needed.
- Using a sterile syringe, slowly inject Sterile Water for Injection, USP, using the volume provided in Table 4, into the POLIVY vial, with the stream directed toward the inside wall of the vial to obtain a concentration of 20 mg/mL of polatuzumab vedotin-piiq.
Table 4 Reconstitution Volumes Strength Volume of Sterile Water for Injection, USP required for reconstitution 30 mg vial 1.8 mL 140 mg vial 7.2 mL - Swirl the vial gently until completely dissolved. Do not shake.
- Inspect the reconstituted solution for discoloration and particulate matter. The reconstituted solution should appear colorless to slightly brown, clear to slightly opalescent, and free of visible particulates. Do not use if the reconstituted solution is discolored, is cloudy, or contains visible particulates. Do not freeze or expose to direct sunlight.
- If needed, store unused reconstituted POLIVY solution refrigerated at 2°C to 8°C (36°F to 46°F) for up to 48 hours or at room temperature (9°C to 25°C, 47°F to 77°F) up to a maximum of 8 hours prior to dilution. Discard vial when cumulative storage time prior to dilution exceeds 48 hours.
Dilution
- Dilute polatuzumab vedotin-piiq to a final concentration of 0.72–2.7 mg/mL in an intravenous infusion bag with a minimum volume of 50 mL containing 0.9% Sodium Chloride Injection, USP, 0.45% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP.
- Determine the volume of 20 mg/mL reconstituted solution needed based on the required dose.
- Withdraw the required volume of reconstituted solution from the POLIVY vial using a sterile syringe and dilute into the intravenous infusion bag. Discard any unused portion left in the vial.
- Gently mix the intravenous bag by slowly inverting the bag. Do not shake.
- Inspect the intravenous bag for particulates and discard if present.
- If not used immediately, store the diluted POLIVY solution as specified in Table 5. Discard if storage time exceeds these limits. Do not freeze or expose to direct sunlight.
Table 5 Diluted POLIVY Solution Storage Conditions Diluent Used to Prepare Solution for Infusion Diluted POLIVY Solution Storage Conditions* - *
- To ensure product stability, do not exceed specified storage durations.
0.9% Sodium Chloride Injection, USP Up to 36 hours at 2°C to 8°C (36°F to 46°F) or up to 4 hours at room temperature (9 to 25°C, 47 to 77°F) 0.45% Sodium Chloride Injection, USP Up to 18 hours at 2°C to 8°C (36°F to 46°F) or up to 4 hours at room temperature (9 to 25°C, 47 to 77°F) 5% Dextrose Injection, USP Up to 36 hours at 2°C to 8°C (36°F to 46°F) or up to 6 hours at room temperature (9 to 25°C, 47 to 77°F) - Limit transportation to 30 minutes at 9°C to 25°C or 24 hours at 2°C to 8°C (refer to instructions below). The total storage plus transportation times of the diluted product should not exceed the storage duration specified in Table 5.
- Agitation stress can result in aggregation. Limit agitation of diluted product during preparation and transportation to administration site. Do not transport diluted product through an automated system (e.g., pneumatic tube or automated cart). If the prepared solution will be transported to a separate facility, remove air from the infusion bag to prevent aggregation. If air is removed, an infusion set with a vented spike is required to ensure accurate dosing during the infusion.
- No incompatibilities have been observed between POLIVY and intravenous infusion bags with product-contacting materials of polyvinyl chloride (PVC) or polyolefins (PO) such as polyethylene (PE) and polypropylene (PP). No incompatibilities have been observed with infusion sets or infusion aids with product-contacting materials of PVC, PE, polyurethane (PU), polybutadiene (PBD), acrylonitrile butadiene styrene (ABS), polycarbonate (PC), polyetherurethane (PEU), fluorinated ethylene propylene (FEP), or polytetrafluorethylene (PTFE), or with filter membranes composed of polyether sulfone (PES) or polysulfone (PSU).
Administration
- Administer POLIVY as an intravenous infusion only.
- Administer the initial dose of POLIVY over 90 minutes. Monitor patients for infusion-related reactions during the infusion and for a minimum of 90 minutes following completion of the initial dose. If the previous infusion was well tolerated, the subsequent dose of POLIVY may be administered as a 30-minute infusion and patients should be monitored during the infusion and for at least 30 minutes after completion of the infusion.
- POLIVY must be administered using a dedicated infusion line equipped with a sterile, non-pyrogenic, low-protein-binding in-line or add-on filter (0.2- or 0.22-micron pore size) and a catheter.
- Do not mix POLIVY with or administer as an infusion with other drugs.
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 30 mg/vial or 140 mg/vial of polatuzumab vedotin-piiq as a white to grayish-white lyophilized powder in a single-dose vial for reconstitution and further dilution.
For injection: 30 mg/vial or 140 mg/vial of polatuzumab vedotin-piiq as a white to grayish-white lyophilized powder in a single-dose vial for reconstitution and further dilution.
Close -
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Peripheral Neuropathy - POLIVY can cause peripheral neuropathy, including severe cases. Peripheral neuropathy occurs as early as the first cycle of treatment and is a cumulative effect [see ...
5.1 Peripheral Neuropathy
POLIVY can cause peripheral neuropathy, including severe cases. Peripheral neuropathy occurs as early as the first cycle of treatment and is a cumulative effect [see Adverse Reactions (6.1)]. POLIVY may exacerbate pre-existing peripheral neuropathy.
In POLARIX, of 435 patients treated with POLIVY plus R-CHP, 53% reported new or worsening peripheral neuropathy, with a median time to onset of 2.3 months. Peripheral neuropathy was Grade 1 in 39% of patients, Grade 2 in 12%, and Grade 3 in 1.6%. Peripheral neuropathy resulted in dose reduction in 4% of treated patients and treatment discontinuation in 0.7%. Among patients with peripheral neuropathy after POLIVY, 58% reported resolution after a median of 4 months.
In Study GO29365, of 173 patients treated with POLIVY, 40% reported new or worsening peripheral neuropathy, with a median time to onset of 2.1 months. Peripheral neuropathy was Grade 1 in 26% of patients, Grade 2 in 12%, and Grade 3 in 2.3%. Peripheral neuropathy resulted in POLIVY dose reduction in 2.9% of treated patients, dose delay in 1.2%, and permanent discontinuation in 2.9%. Sixty-five percent of patients reported improvement or resolution of peripheral neuropathy after a median of 1 month, and 48% reported complete resolution.
The peripheral neuropathy is predominantly sensory; however, motor and sensorimotor peripheral neuropathy also occur. Monitor for symptoms of peripheral neuropathy such as hypoesthesia, hyperesthesia, paresthesia, dysesthesia, neuropathic pain, burning sensation, weakness, or gait disturbance. Patients experiencing new or worsening peripheral neuropathy may require a delay, dose reduction, or discontinuation of POLIVY [see Dosage and Administration (2.2)].
5.2 Infusion-Related Reactions
POLIVY can cause infusion-related reactions, including severe cases. Delayed infusion-related reactions as late as 24 hours after receiving POLIVY have occurred.
With premedication, 13% of patients (58/435) in POLARIX reported infusion-related reactions after the administration of POLIVY plus R-CHP. The reactions were Grade 1 in 4.4% of patients, Grade 2 in 8%, and Grade 3 in 1.1%.
With premedication, 7% of patients (12/173) in Study GO29365 reported infusion-related reactions after the administration of POLIVY. The reactions were Grade 1 in 4.6% of patients, Grade 2 in 1.7%, and Grade 3 in 0.6%.
Symptoms occurring in ≥1% of patients included chills, dyspnea, pyrexia, pruritus, rash, and chest discomfort. Administer an antihistamine and antipyretic prior to the administration of POLIVY, and monitor patients closely throughout the infusion. If an infusion-related reaction occurs, interrupt the infusion and institute appropriate medical management [see Dosage and Administration (2.2)].
5.3 Myelosuppression
Treatment with POLIVY can cause serious or severe myelosuppression, including neutropenia, thrombocytopenia, and anemia.
In POLARIX, 90% of patients treated with POLIVY plus R-CHP had primary prophylaxis with G-CSF. Grade 3–4 hematologic adverse reactions included lymphopenia (44%), neutropenia (39%), febrile neutropenia (15%), anemia (14%), and thrombocytopenia (8%) [see Adverse Reactions (6.1)].
In Study GO29365, in patients treated with POLIVY plus BR (n = 45), 42% received primary prophylaxis with G-CSF. Grade 3 or higher hematologic adverse reactions included neutropenia (42%), thrombocytopenia (40%), anemia (24%), lymphopenia (13%), and febrile neutropenia (11%) [see Adverse Reactions (6.1)]. Grade 4 hematologic adverse reactions included neutropenia (24%), thrombocytopenia (16%), lymphopenia (9%), and febrile neutropenia (4.4%). Cytopenias were the most common reason for treatment discontinuation (18% of all patients).
Monitor complete blood counts throughout treatment. Cytopenias may require a delay, dose reduction, or discontinuation of POLIVY [see Dosage and Administration (2.2)]. Administer prophylactic G-CSF for neutropenia in patients receiving POLIVY plus R-CHP. Consider prophylactic G-CSF administration in patients receiving POLIVY plus bendamustine and a rituximab product.
5.4 Serious and Opportunistic Infections
Fatal and/or serious infections, including opportunistic infections such as sepsis, pneumonia (including Pneumocystis jiroveci and other fungal pneumonia), herpesvirus infection, and cytomegalovirus infection have occurred in patients treated with POLIVY [see Adverse Reactions (6.1)].
In POLARIX, Grade 3–4 infections occurred in 14% (61/435) of patients treated with POLIVY plus R-CHP and infection-related deaths were reported in 1.1% of patients.
In Study GO29365, Grade 3 or higher infections occurred in 32% (55/173) of patients treated with POLIVYand infection-related deaths were reported in 2.9% of patients within 90 days of last treatment.
Closely monitor patients during treatment for signs of infection. Administer prophylaxis for Pneumocystis jiroveci pneumonia and herpesvirus. Administer prophylactic G-CSF for neutropenia as recommended [see Dosage and Administration (2.3)].
5.5 Progressive Multifocal Leukoencephalopathy (PML)
PML has been reported after treatment with POLIVY plus bendamustine and obinutuzumab in study GO29365 (0.6%, 1/173). Monitor for new or worsening neurological, cognitive, or behavioral changes. Hold POLIVY and any concomitant chemotherapy if PML is suspected, and permanently discontinue if the diagnosis is confirmed.
5.6 Tumor Lysis Syndrome
POLIVY may cause tumor lysis syndrome. Patients with high tumor burden and rapidly proliferative tumor may be at increased risk of tumor lysis syndrome. Monitor closely and take appropriate measures, including tumor lysis syndrome prophylaxis.
5.7 Hepatotoxicity
Serious cases of hepatotoxicity that were consistent with hepatocellular injury, including elevations of transaminases and/or bilirubin, have occurred in patients treated with POLIVY.
In recipients of POLIVY plus R-CHP, Grade 3–4 elevation of ALT and AST developed in 1.4% and 0.7% of patients, respectively.
In Study GO29365, Grade 3 and Grade 4 transaminase elevations each developed in 1.9% of patients.
Preexisting liver disease, elevated baseline liver enzymes, and concomitant medications may increase the risk of hepatotoxicity. Monitor liver enzymes and bilirubin level.
Close5.8 Embryo-Fetal Toxicity
Based on the mechanism of action and findings from animal studies, POLIVY can cause fetal harm when administered to a pregnant woman. The small molecule component of POLIVY, MMAE, administered to rats caused adverse developmental outcomes, including embryo-fetal mortality and structural abnormalities, at exposures below those occurring clinically at the recommended dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with POLIVY and for 3 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with POLIVY and for 5 months after the last dose [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the label: Peripheral Neuropathy [see Warnings and Precautions (5.1)] Infusion-Related ...
The following clinically significant adverse reactions are discussed in greater detail in other sections of the label:
- Peripheral Neuropathy [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Myelosuppression [see Warnings and Precautions (5.3)]
- Serious and Opportunistic Infections [see Warnings and Precautions (5.4)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.5)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
Close6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure to POLIVY 1.8 mg/kg in 480 patients with large B-cell lymphoma (LBCL), including those with previously untreated LBCL (POLARIX) and relapsed or refractory DLBCL (GO29365).
Previously Untreated DLBCL, NOS or HGBL
GO39942 (POLARIX)
The safety of POLIVY in combination with R-CHP chemoimmunotherapy was evaluated in POLARIX, a randomized double-blind, placebo-controlled, multicenter study of 873 patients with previously untreated large B-cell lymphoma, 435 of whom received POLIVY plus R-CHP [see Clinical Studies (14.1)].
Patients were randomized 1:1 to receive POLIVY plus R-CHP or to receive R-CHOP for six 21-day cycles followed by two additional cycles of rituximab alone in both arms. Granulocyte colony-stimulating factor (G-CSF) primary prophylaxis was required and administered to 90% of patients in the POLIVY plus R-CHP arm and 93% of patients in the R-CHOP arm. Following premedication with an antihistamine and antipyretic, POLIVY was administered intravenously at 1.8 mg/kg on Day 1 of Cycles 1–6. R-CHP was administered starting on Day 1 of Cycles 1–6. Rituximab monotherapy was administered on Day 1 of Cycles 7–8 [see Clinical Studies (14.1)].
The trial required an absolute neutrophil count ≥1,000/µL, platelet count ≥75,000/µL, creatinine clearance (CLcr) ≥40 mL/min, hepatic transaminases ≤2.5 times the upper limit of normal (ULN), and bilirubin <1.5 times ULN, unless abnormalities were from the underlying disease. The trial excluded patients having age >80, ECOG performance status above 2, known central nervous system (CNS) lymphoma, and Grade 2 or higher peripheral neuropathy.
The median age was 65 years overall (range: 19 to 80 years); 54% of patients were male; 53% were White, 19% were Asian, 2%, Black or African American, and 5% were Hispanic or Latino.
In the POLIVY plus R-CHP group, 92% of patients received 6 cycles of POLIVY, and 94% completed 6 cycles of combination therapy.
Serious adverse reactions occurred in 34% of patients who received POLIVY plus R-CHP, including febrile neutropenia and pneumonia in ≥5% of recipients. Fatal adverse reactions occurred in 3% of recipients of POLIVY plus R-CHP within 90 days of last treatment, primarily from infection including pneumonia (0.9%) and sepsis (0.2%).
Adverse reactions led to dose reduction of POLIVY in 6% of patients, mainly from peripheral neuropathy. Adverse reactions lead to dose interruption of POLIVY in 18% of patients, most commonly from pneumonia and neutropenia, and permanent discontinuation of POLIVY in 4.4% of patients.
Table 6 summarizes adverse reactions in POLARIX. In recipients of POLIVY plus R-CHP, adverse reactions in ≥20% of patients, excluding laboratory abnormalities, were peripheral neuropathy, nausea, fatigue, diarrhea, constipation, alopecia, and mucositis. New or worsening Grade 3 to 4 laboratory abnormalities in ≥10% of patients were lymphopenia, neutropenia, hyperuricemia, and anemia.
Table 6 Select Adverse Reactions Occurring in ≥10% of Patients Treated with POLIVY Plus R-CHP in POLARIX Adverse Reactions by Body System POLIVY + R-CHP
n = 435R-CHOP
n = 438All Grades,
%Grade 3–4,
%All Grades,
%Grade 3–4,
%The table includes a combination of grouped and ungrouped terms. Events were graded using NCI CTCAE version 4.0. - *
- Laboratory values are based on integrated analysis of laboratory and adverse reaction data. Reported investigations exclude electrolytes.
- †
- Febrile neutropenia includes febrile neutropenia, febrile bone marrow aplasia, and neutropenic sepsis.
- ‡
- At last assessment, peripheral neuropathy was unresolved in 42% in the POLIVY + R-CHP arm and in 33% in the R-CHOP arm.
- §
- Peripheral neuropathy includes all terms containing "neuropathy", neuralgia, dysesthesia, paresthesia, hypoesthesia, peroneal nerve palsy, hypotonia, hyporeflexia, neuromyopathy, and hyperesthesia.
- ¶
- Mucositis includes stomatitis, oropharyngeal pain, mucosal inflammation, mouth ulceration, oral pain, oropharyngeal discomfort, aphthous ulcer, odynophagia, oral discomfort, tongue blistering, and tongue ulceration.
- #
- Abdominal pain includes abdominal pain, abdominal discomfort, gastrointestinal pain, epigastric discomfort, and related terms.
- Þ
- Edema includes edema, face edema, swelling face, edema peripheral, fluid overload, fluid retention, pulmonary edema, peripheral swelling, and swelling.
- ß
- Infusion related reaction is reflective of the combination regimen due to same-day administration.
- à
- Rash includes rash, dermatitis, and related terms.
- è
- Musculoskeletal pain includes musculoskeletal pain, back pain, musculoskeletal chest pain, neck pain, myalgia, and bone pain.
- ð
- Upper respiratory tract infection incudes sinusitis, laryngitis, pharyngitis, nasopharyngitis, rhinitis, and specific infections.
Blood and Lymphatic System Disorders* Lymphopenia 80 44 77 44 Anemia 68 14 67 11 Neutropenia 60 39 60 42 Thrombocytopenia 32 8 33 6 Febrile neutropenia† 15 15 9 9 Investigations* Creatinine increased 66 0.7 64 0.9 Aspartate aminotransferase increased 26 0.7 23 1.1 Alanine aminotransferase increased 25 1.4 27 0.5 Alkaline phosphatase increased 23 0 22 0.5 Uric acid increased 19 18 17 16 Weight decreased 13 0.9 12 0.2 Nervous System Disorders Peripheral neuropathy‡,§ 53 1.6 54 1.1 Altered taste 14 0 16 0 Headache 13 0.2 14 0.9 Gastrointestinal Disorders Nausea 42 1.1 37 0.5 Diarrhea 31 3.9 20 1.8 Constipation 29 1.1 29 0.2 Mucositis¶ 22 1.4 19 0.5 Abdominal pain# 16 1.1 14 1.6 Vomiting 15 1.1 14 0.7 General Disorders Fatigue 37 2.5 38 3.0 Pyrexia 16 1.4 13 0 EdemaÞ 14 0.5 11 0.2 Infusion-related reactionß 13 1.1 16 1.6 Skin and Subcutaneous Tissue Disorders Alopecia 24 0 24 0.2 Rashà 13 0.7 11 0 Musculoskeletal Disorders Musculoskeletal painè 19 0.5 21 1.8 Infections Upper respiratory tract infectionð 17 0.5 16 0.5 Metabolism and Nutrition Disorders Decreased appetite 17 1.1 14 0.7 Respiratory Disorders Cough 15 0 14 0 Dyspnea 13 0.9 10 0.9 Relapsed or Refractory DLBCL, NOS
GO29365
The data described in this section reflect exposure to POLIVY in Study GO29365, a multicenter clinical trial for adult patients with relapsed or refractory B-cell lymphomas [see Clinical Studies (14.2)]. In patients with relapsed or refractory DLBCL, the trial included a single-arm safety evaluation of POLIVY in combination with bendamustine and a rituximab product (BR) (n = 6), followed by an open-label randomization to POLIVY in combination with BR versus BR alone (n = 39 treated per arm).
Following premedication with an antihistamine and antipyretic, POLIVY 1.8 mg/kg was administered by intravenous infusion on Day 2 of Cycle 1 and on Day 1 of Cycles 2–6, with a cycle length of 21 days. Bendamustine 90 mg/m2 daily was administered intravenously on Days 2 and 3 of Cycle 1 and on Days 1 and 2 of Cycles 2–6. A rituximab product dosed at 375 mg/m2 was administered intravenously on Day 1 of each cycle. Granulocyte colony-stimulating factor primary prophylaxis was optional and administered to 42% of recipients of POLIVY plus BR.
In POLIVY-treated patients (n = 45), the median age was 67 years (range 33 – 86) with 58% being ≥age 65, 69% were male, 69% were White, and 87% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. The trial required an absolute neutrophil count ≥1500/µL, platelet count ≥75/µL, creatinine clearance (CLcr) ≥40 mL/min, hepatic transaminases ≤2.5 times ULN, and bilirubin <1.5 times ULN, unless abnormalities were from the underlying disease. Patients with Grade 2 or higher peripheral neuropathy or prior allogeneic hematopoietic stem cell transplantation (HSCT) were excluded.
Patients treated with POLIVY plus BR received a median of 5 cycles, with 49% receiving 6 cycles. Patients treated with BR alone received a median of 3 cycles, with 23% receiving 6 cycles.
Fatal adverse reactions occurred in 7% of recipients of POLIVY plus BR within 90 days of last treatment. Serious adverse reactions occurred in 64%, most often from infection. Serious adverse reactions in ≥5% of recipients of POLIVY plus BR included pneumonia (16%), febrile neutropenia (11%), pyrexia (9%), and sepsis (7%).
In recipients of POLIVY plus BR, adverse reactions led to dose reduction in 18%, dose interruption in 51%, and permanent discontinuation of all treatment in 31%. The most common adverse reactions leading to treatment discontinuation were thrombocytopenia and/or neutropenia.
Table 7 summarizes commonly reported adverse reactions. In recipients of POLIVY plus BR, adverse reactions in ≥20% of patients included neutropenia, thrombocytopenia, anemia, peripheral neuropathy, fatigue, diarrhea, pyrexia, decreased appetite, and pneumonia.
Table 7 Adverse Reactions Occurring in >10% of Patients with Relapsed or Refractory DLBCL and ≥5% More in the POLIVY Plus Bendamustine and Rituximab Product Group in Study GO29365 Adverse Reactions by Body System POLIVY + BR
n = 45BR
n = 39All Grades,
%Grade 3 or Higher,
%All Grades,
%Grade 3 or Higher,
%The table includes a combination of grouped and ungrouped terms. Events were graded using NCI CTCAE version 4. Blood and Lymphatic System Disorders Neutropenia 49 42 44 36 Thrombocytopenia 49 40 33 26 Anemia 47 24 28 18 Lymphopenia 13 13 8 8 Nervous System Disorders Peripheral neuropathy 40 0 8 0 Dizziness 13 0 8 0 Gastrointestinal Disorders Diarrhea 38 4.4 28 5 Vomiting 18 2.2 13 0 General Disorders Infusion-related reaction 18 2.2 8 0 Pyrexia 33 2.2 23 0 Decreased appetite 27 2.2 21 0 Infections Pneumonia 22 16* 15 2.6† Upper respiratory tract infection 13 0 8 0 Investigations Weight decreased 16 2.2 8 2.6 Metabolism and Nutrition Disorders Hypokalemia 16 9 10 2.6 Hypoalbuminemia 13 2.2 8 0 Hypocalcemia 11 2.2 5 0 Other clinically relevant adverse reactions (<10% or with a <5% difference) in recipients of POLIVY plus BR included:
- Blood and lymphatic system disorders: pancytopenia (7%)
- Musculoskeletal disorders: arthralgia (7%)
- Investigations: hypophosphatemia (9%), transaminase elevation (7%), lipase increased (7%)
- Respiratory disorders: pneumonitis (4.4%)
Selected treatment-emergent laboratory abnormalities are summarized in Table 8. In recipients of POLIVY plus BR, >20% of patients developed Grade 3 or 4 neutropenia, leukopenia, or thrombocytopenia, and >10% developed Grade 4 neutropenia (13%) or Grade 4 thrombocytopenia (11%).
Table 8 Select Laboratory Abnormalities Worsening from Baseline in Patients with Relapsed or Refractory DLBCL and ≥5% More in the POLIVY Plus Bendamustine and Rituximab Product Group Laboratory Parameter* POLIVY + BR
n = 45BR
n = 39All Grades,
(%)Grade 3–4,
(%)All Grades,
(%)Grade 3–4,
(%)- *
- Includes laboratory abnormalities that are new or worsening in grade or with worsening from baseline unknown.
Hematologic Lymphocyte count decreased 87 87 90 82 Neutrophil count decreased 78 61 56 33 Hemoglobin decreased 78 18 62 10 Platelet count decreased 76 31 64 26 Chemistry Creatinine increased 87 4.4 77 5 Calcium decreased 44 9 26 0 SGPT/ALT increased 38 0 8 2.6 SGOT/AST increased 36 0 26 2.6 Lipase increased 36 9 13 5 Phosphorus decreased 33 7 28 8 Amylase increased 24 0 18 2.6 Potassium decreased 24 11 28 5 Safety was also evaluated in 173 adult patients with relapsed or refractory lymphoma who received POLIVY, bendamustine, and either a rituximab product or obinutuzumab in Study GO29365, including the 45 patients with DLBCL described above. In the expanded safety population, the median age was 66 years (range 27 – 86), 57% were male, 91% had an ECOG performance status of 0-1, and 32% had a history of peripheral neuropathy at baseline.
Fatal adverse reactions occurred in 4.6% of recipients of POLIVY within 90 days of last treatment, with infection as a leading cause. Serious adverse reactions occurred in 60%, most often from infection.
Table 9 summarizes the most common adverse reactions in the expanded safety population. The overall safety profile was similar to that described above. Adverse reactions in ≥20% of patients were diarrhea, neutropenia, peripheral neuropathy, fatigue, thrombocytopenia, pyrexia, decreased appetite, anemia, and vomiting. Infection-related adverse reactions in >10% of patients included upper respiratory tract infection, febrile neutropenia, pneumonia, and herpesvirus infection.
Table 9 Most Common Adverse Reactions (≥20% Any Grade or ≥5% Grade 3 or Higher) in Recipients of POLIVY and Chemoimmunotherapy for Relapsed or Refractory Lymphoma Adverse Reaction by Body System POLIVY + Bendamustine + Rituximab Product or Obinutuzumab
n = 173All Grades,
%Grade 3 or Higher,
%The table includes a combination of grouped and ungrouped terms. Blood and Lymphatic System Disorders Neutropenia 44 39 Thrombocytopenia 31 23 Anemia 28 14 Febrile neutropenia* 13 13 Leukopenia 13 8 Lymphopenia 12 12 Nervous System Disorders Peripheral neuropathy 40 2.3 Gastrointestinal Disorders Diarrhea 45 8 Vomiting 27 2.9 General Disorders Fatigue 40 5 Pyrexia 30 2.9 Decreased appetite 29 1.7 Infections Pneumonia 13 10† Sepsis 6 6‡ Metabolism and Nutrition Disorders Hypokalemia 18 6 Other clinically relevant adverse reactions (<20% any grade) included:
- General disorders: infusion-related reaction (7%)
- Infection: upper respiratory tract infection (16%), lower respiratory tract infection (10%), herpesvirus infection (12%), cytomegalovirus infection (1.2%)
- Respiratory: dyspnea (19%), pneumonitis (1.7%)
- Nervous system disorders: dizziness (10%)
- Investigations: weight decrease (10%), transaminase elevation (8%), lipase increase (3.5%)
- Musculoskeletal disorders: arthralgia (7%)
- Eye disorders: blurred vision (1.2%)
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on POLIVY - Strong CYP3A Inhibitors - Concomitant use with a strong CYP3A4 inhibitor may increase unconjugated MMAE AUC [see Clinical Pharmacology (12.3)], which ...Close
7.1 Effects of Other Drugs on POLIVY
Strong CYP3A Inhibitors
Concomitant use with a strong CYP3A4 inhibitor may increase unconjugated MMAE AUC [see Clinical Pharmacology (12.3)], which may increase POLIVY toxicities. Monitor patients for signs of toxicity.
Strong CYP3A Inducers
Concomitant use with a strong CYP3A4 inducer may decrease unconjugated MMAE AUC [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], POLIVY can cause fetal harm. There are no available data in ...
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], POLIVY can cause fetal harm. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of the small molecule component of POLIVY, MMAE, to pregnant rats during organogenesis at exposures below the clinical exposure at the recommended dose of 1.8 mg/kg POLIVY every 21 days resulted in embryo-fetal mortality and structural abnormalities (see Data). Advise a pregnant woman of the potential risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
No embryo-fetal development studies in animals have been performed with polatuzumab vedotin-piiq. In an embryo-fetal developmental study in pregnant rats, administration of two intravenous doses of MMAE, the small molecule component of POLIVY, on gestational days 6 and 13 caused embryo-fetal mortality and structural abnormalities, including protruding tongue, malrotated limbs, gastroschisis, and agnathia compared to controls at a dose of 0.2 mg/kg (approximately 0.5-fold the human area under the curve [AUC] at the recommended dose).
8.2 Lactation
Risk Summary
There is no information regarding the presence of polatuzumab vedotin-piiq in human milk, the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with POLIVY and for 2 months after the last dose.
8.3 Females and Males of Reproductive Potential
POLIVY can cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating POLIVY [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with POLIVY and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with POLIVY and for 5 months after the final dose [see Nonclinical Toxicity (13.1)].
Infertility
Females
Based on findings in animal studies with MMAE-containing antibody-drug conjugates (ADCs), POLIVY may impair female fertility. The effect on fertility is reversible [see Nonclinical Toxicology (13.1)].
Males
Based on findings from animal studies, POLIVY may impair male fertility. The reversibility of this effect is unknown [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of POLIVY have not been established in pediatric patients.
8.5 Geriatric Use
Among 435 patients treated with POLIVY plus R-CHP in POLARIX, 227 (52%) were ≥65 years of age. No overall differences in safety or efficacy were observed between patients aged ≥65 years and younger patients.
Among 173 patients treated with POLIVY plus BR in Study GO29365, 95 (55%) were ≥65 years of age. Patients aged ≥65 had a numerically higher incidence of serious adverse reactions (64%) than patients aged <65 (53%). This study did not include sufficient numbers of patients to determine whether efficacy differed in patients aged ≥65 and younger patients.
Close8.6 Hepatic Impairment
Avoid the administration of POLIVY in patients with moderate or severe hepatic impairment (total bilirubin greater than 1.5 × ULN and any AST). Patients with moderate or severe hepatic impairment are likely to have increased exposure to MMAE, which may increase the risk of adverse reactions. POLIVY has not been studied in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3) and Warnings and Precautions (5.7)].
No adjustment in the starting dose is required when administering POLIVY to patients with mild hepatic impairment (total bilirubin 1 to 1.5 × ULN or AST greater than ULN).
-
11 DESCRIPTIONPolatuzumab vedotin-piiq is a CD79b-directed antibody and microtubule inhibitor conjugate. It consists of three components: 1) the humanized immunoglobulin G1 (IgG1) monoclonal antibody specific ...
Polatuzumab vedotin-piiq is a CD79b-directed antibody and microtubule inhibitor conjugate. It consists of three components: 1) the humanized immunoglobulin G1 (IgG1) monoclonal antibody specific for human CD79b; 2) the small molecule anti-mitotic agent MMAE; and 3) a protease-cleavable linker maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (mc-vc-PAB) that covalently attaches MMAE to the polatuzumab antibody.
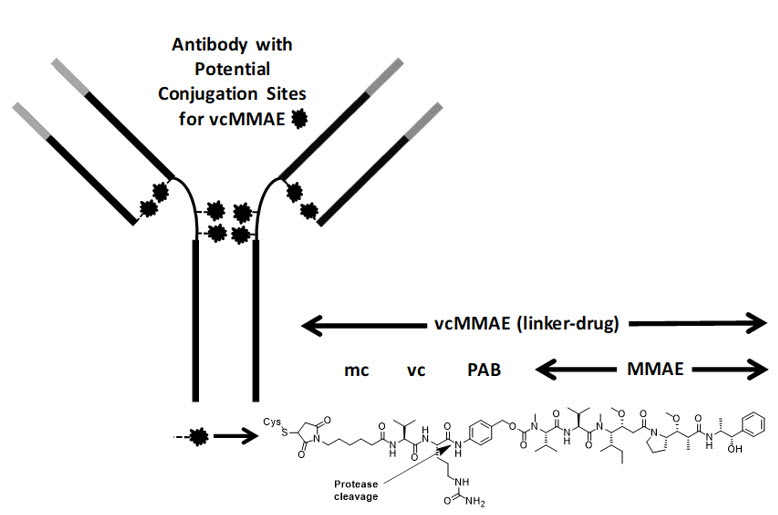
Polatuzumab vedotin-piiq has an approximate molecular weight of 150 kDa. An average of 3.5 molecules of MMAE are attached to each antibody molecule. Polatuzumab vedotin-piiq is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the small molecule components are produced by chemical synthesis.
POLIVY (polatuzumab vedotin-piiq) for injection is supplied as a sterile, white to grayish-white, preservative-free, lyophilized powder, which has a cake-like appearance, for intravenous infusion after reconstitution and dilution.
Each single-dose 30 mg POLIVY vial delivers 30 mg of polatuzumab vedotin-piiq, polysorbate-20 (1.8 mg), sodium hydroxide (0.82 mg), succinic acid (1.77 mg), and sucrose (62 mg). After reconstitution with 1.8 mL of Sterile Water for Injection, USP, the final concentration is 20 mg/mL with a pH of approximately 5.3.
Each single-dose 140 mg POLIVY vial delivers 140 mg of polatuzumab vedotin-piiq, polysorbate-20 (8.4 mg), sodium hydroxide (3.80 mg), succinic acid (8.27 mg), and sucrose (288 mg). After reconstitution with 7.2 mL of Sterile Water for Injection, USP, the final concentration is 20 mg/mL with a pH of approximately 5.3.
The POLIVY vial stoppers are not made with natural rubber latex.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Polatuzumab vedotin-piiq is a CD79b-directed antibody-drug conjugate with activity against dividing B cells. The small molecule, MMAE, is an anti-mitotic agent ...
12.1 Mechanism of Action
Polatuzumab vedotin-piiq is a CD79b-directed antibody-drug conjugate with activity against dividing B cells. The small molecule, MMAE, is an anti-mitotic agent covalently attached to the antibody via a cleavable linker. The monoclonal antibody binds to CD79b, a B-cell specific surface protein, which is a component of the B-cell receptor. Upon binding CD79b, polatuzumab vedotin-piiq is internalized, and the linker is cleaved by lysosomal proteases to enable intracellular delivery of MMAE. MMAE binds to microtubules and kills dividing cells by inhibiting cell division and inducing apoptosis.
12.2 Pharmacodynamics
Over polatuzumab vedotin-piiq dosages of 0.1 to 2.4 mg/kg (0.06 to 1.33 times the approved recommended dosage), higher exposures (AUC and Cmax) of antibody-conjugated MMAE (acMMAE) and unconjugated MMAE were associated with higher incidence of some adverse reactions (including ≥Grade 3 thrombocytopenia and ≥Grade 3 anemia). Higher exposure (AUC and Cmax) of unconjugated MMAE was associated with higher incidence of ≥Grade 2 peripheral neuropathy. Lower acMMAE exposure (AUC) was associated with lower efficacy in patients with relapsed or refractory DLBCL.
12.3 Pharmacokinetics
The exposure parameters of acMMAE and unconjugated MMAE (the cytotoxic component of polatuzumab vedotin-piiq) are summarized in Table 10. The plasma exposure of acMMAE and unconjugated MMAE increased proportionally over a polatuzumab vedotin-piiq dose range from 0.1 to 2.4 mg/kg (0.06 to 1.33 times the approved recommended dosage). Cycle 3 acMMAE AUC were predicted to increase by approximately 30% over Cycle 1 AUC, and achieved more than 90% of the Cycle 6 AUC. Unconjugated MMAE plasma exposures were <3% of acMMAE exposures, and the AUC and Cmax were predicted to decrease after repeated every-3-week dosing.
Table 10 Cycle 1 Exposure of acMMAE and Unconjugated MMAE*,† R/R DLBCL, NOS Previously Untreated DLBCL, NOS or HGBL acMMAE Unconjugated MMAE acMMAE Unconjugated MMAE Cmax=maximum concentration; AUC=area under the concentration-time curve from time zero to day 21. Cmax (ng/mL) 688 (15%) 3.19 (57%) 587 (15%) 2.45 (46%) AUC (day*ng/mL) 2040 (35%) 31.0 (56%) 1690 (22%) 20.8 (50%) Distribution
The acMMAE central volume of distribution is 3.15 L. For humans, MMAE plasma protein binding is 71% to 77% and the blood-to-plasma ratio is 0.79 to 0.98, in vitro.
Elimination
At the end of Cycle 6, the median (min, max) acMMAE terminal half-life was 12.2 days (4.5 to 36.7 days) and the clearance was 0.9 L/day in patients with B-cell malignancies. The median (min, max) unconjugated MMAE terminal half-life was 3.74 days (1.58 to 10.1 days) days after the first polatuzumab vedotin-piiq dose.
Metabolism
Polatuzumab vedotin-piiq catabolism has not been studied in humans; however, it is expected to undergo catabolism to small peptides, amino acids, unconjugated MMAE, and unconjugated MMAE-related catabolites. MMAE is a substrate for CYP3A4.
Specific Populations
No clinically significant differences in the pharmacokinetics of acMMAE or unconjugated MMAE were observed based on age (19 to 89 years), sex (males versus females), race (White 69%, Asian 11%), or mild to moderate renal impairment (CLcr 30 to 89 mL/min).
The effect of severe renal impairment (CLcr 15 to 29 mL/min) or end-stage renal disease with or without dialysis on the pharmacokinetics of acMMAE or unconjugated MMAE is unknown.
Patients with Hepatic Impairment
Compared to patients with normal hepatic function, geometric mean MMAE exposure was 11% higher in patients with previously untreated DLBCL and mild hepatic impairment (total bilirubin 1 to 1.5 × ULN or any AST greater than ULN) and 40% higher in patients with relapsed or refractory DLBCL and mild hepatic impairment. The effect of mild hepatic impairment on MMAE exposure is not expected to have a clinically significant impact.
Mild hepatic impairment was not associated with a significant difference in acMMAE exposure. The effect of moderate to severe hepatic impairment (total bilirubin greater than 1.5 × ULN and any AST) or liver transplantation on the pharmacokinetics of acMMAE or unconjugated MMAE is unknown.
Drug Interaction Studies
No dedicated clinical drug-drug interaction studies with POLIVY in humans have been conducted.
Physiologically-Based Pharmacokinetic (PBPK) Modeling Predictions:
Strong CYP3A Inhibitor: Concomitant use of polatuzumab vedotin-piiq with ketoconazole (strong CYP3A inhibitor) is predicted to increase unconjugated MMAE AUC by 45%.
Population Pharmacokinetic (popPK) Modeling Predictions:
Bendamustine or Rituximab: No clinically significant differences in the pharmacokinetics of acMMAE or unconjugated MMAE are predicted when polatuzumab vedotin-piiq is used concomitantly with bendamustine or rituximab.
Rituximab, Cyclophosphamide, Doxorubicin, or Prednisone (R-CHP): No clinically significant differences in the pharmacokinetics of acMMAE or unconjugated MMAE are predicted when polatuzumab vedotin-piiq is used concomitantly with R-CHP.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of POLIVY or of other polatuzumab products.
In studies POLARIX and GO29365, 1.4% (6/427) and 6% (8/134) of patients tested positive for antibodies against polatuzumab vedotin-piiq, respectively, of which none were positive for neutralizing antibodies. Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of polatuzumab products is unknown.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies in animals have not been performed with polatuzumab vedotin-piiq or MMAE. MMAE was positive for genotoxicity in ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in animals have not been performed with polatuzumab vedotin-piiq or MMAE.
MMAE was positive for genotoxicity in the in vivo rat bone marrow micronucleus study through an aneugenic mechanism. MMAE was not mutagenic in the bacterial reverse mutation (Ames) assay or the L5178Y mouse lymphoma forward mutation assay.
Fertility studies in animals have not been performed with polatuzumab vedotin-piiq or MMAE. However, results of repeat-dose toxicity indicate the potential for polatuzumab vedotin-piiq to impair female and male fertility. In the 4-week repeat-dose toxicity study in rats with weekly dosing of 2, 6, and 10 mg/kg, dose-dependent testicular seminiferous tubule degeneration with abnormal lumen contents in the epididymis was observed. Findings in the testes and epididymis did not reverse and correlated with decreased testes weight and gross findings of small and/or soft testes at recovery necropsy in males given doses ≥2 mg/kg (below the exposure at the recommended dose based on unconjugated MMAE AUC).
MMAE-containing ADCs have been associated with adverse ovarian effects when administered to sexually immature animals. Adverse effects included decrease in, or absence of, secondary and tertiary ovarian follicles after weekly administration to cynomolgus monkeys in studies of 4-week duration. These effects showed a trend towards recovery 6 weeks after the end of dosing; no changes were observed in primordial follicles.
-
14 CLINICAL STUDIES14.1 Previously Untreated DLBCL, NOS or HGBL - GO39942 (POLARIX) The efficacy of POLIVY was evaluated in POLARIX (NCT03274492), a randomized double-blind, placebo-controlled, multicenter ...
14.1 Previously Untreated DLBCL, NOS or HGBL
GO39942 (POLARIX)
The efficacy of POLIVY was evaluated in POLARIX (NCT03274492), a randomized double-blind, placebo-controlled, multicenter trial in patients with previously untreated large B-cell lymphoma. Eligible patients were aged 18–80 and had an International Prognostic Index (IPI) score of 2–5 and ECOG performance status of 0–2. The study excluded patients with transformed lymphoma, primary mediastinal large B-cell lymphoma, known CNS lymphoma, or Grade 2 or higher peripheral neuropathy.
Patients were randomized in a 1:1 ratio to receive POLIVY plus R-CHP or to receive R-CHOP for six 21-day cycles followed by two additional cycles of rituximab alone in both arms. Randomization was stratified by IPI score (2 vs 3–5), presence or absence of bulky disease (lesion ≥7.5 cm), and geographical region. Dosing in each treatment arm was as follows:
- POLIVY + R-CHP arm: POLIVY 1.8 mg/kg intravenously, rituximab 375 mg/m2 intravenously, cyclophosphamide 750 mg/m2 intravenously, and doxorubicin 50 mg/m2 intravenously on Day 1 and prednisone 100 mg orally once daily on Days 1–5 for 6 cycles. Rituximab 375 mg/m2 was administered intravenously on Day 1 of cycles 7 and 8.
- R-CHOP arm: rituximab 375 mg/m2 intravenously, cyclophosphamide 750 mg/m2 intravenously, doxorubicin 50 mg/m2 intravenously, and vincristine 1.4 mg/m2 intravenously on Day 1 and prednisone 100 mg orally once daily on Days 1–5 for 6 cycles. Rituximab 375 mg/m2 was administered intravenously on Day 1 of cycles 7 and 8.
Prophylaxis with granulocyte-colony stimulating factor (G-CSF) was mandated for both arms. Dosing in both treatment arms was preceded by premedication.
Of the 879 patients randomized (440 to POLIVY plus R-CHP, 439 to R-CHOP), the median age was 65 years (range 19 to 80 years), 54% were male, 54% were White, 19% were Asian, 1.8% were Black or African American, and 6% were Hispanic or Latino. In total, 38% had an IPI score of 2, 62% had an IPI score of 3–5, 89% had Stage 3 or 4 disease, and 44% had bulky disease. The majority of patients had DLBCL, NOS (84%; n = 740), 11% (n = 93) had HGBL with MYC and BCL2 and/or BCL6 rearrangements or HGBL, NOS, and 5% had other large B-cell lymphomas.
Efficacy was based on investigator-assessed progression-free survival (PFS). Other efficacy measures included modified event-free survival. Efficacy results are summarized in Table 11 and in Figure 1.
Table 11 Summary of Efficacy in POLARIX by Intention-to-Treat Analysis Outcomes POLIVY + R-CHP
n = 440R-CHOP
n = 439CI=confidence interval; CR=complete response; EFS=event-free survival; HR=hazard ratio; PFS=progression-free survival. - *
- Estimated median follow-up for PFS was 24.7 months in both arms combined.
- †
- Stratified log-rank test, with a two-sided significance boundary of 0.05. The hierarchical testing order was PFS, modified EFS, then CR rate and overall survival.
- ‡
- Modified EFS was defined as time from randomization to the earliest occurrence of disease progression or relapse, death, an efficacy finding that led to non-protocol specified lymphoma treatment, or biopsy positive for residual disease.
- §
- By blinded independent central review, per 2014 Lugano response criteria.
- ¶
- Cochran-Mantel-Haenszel chi-squared test, with a two-sided significance boundary of 0.01.
Progression-Free Survival per Investigator* Number (%) of patients with event 107 (24) 134 (31) Progression 88 114 Death 19 20 HR (95% CI) 0.73 (0.57, 0.95) p-value† 0.0177 Modified Event-Free Survival per Investigator‡ Number (%) of patients with event 112 (26) 138 (31) HR (95% CI) 0.75 (0.58, 0.96) p-value† 0.0244 Objective Response at End of Treatment§ Objective response rate, % (95% CI) 86 (82, 89) 84 (80, 87) CR rate, % 78 (74, 82) 74 (70, 78) Difference in CR rate, % (95% CI) 3.9 (–1.9, 9.7) p-value¶ 0.1557 Figure 1 Kaplan-Meier Curve of Investigator-Assessed Progression-Free Survival in POLARIX by Intention-to-Treat Analysis
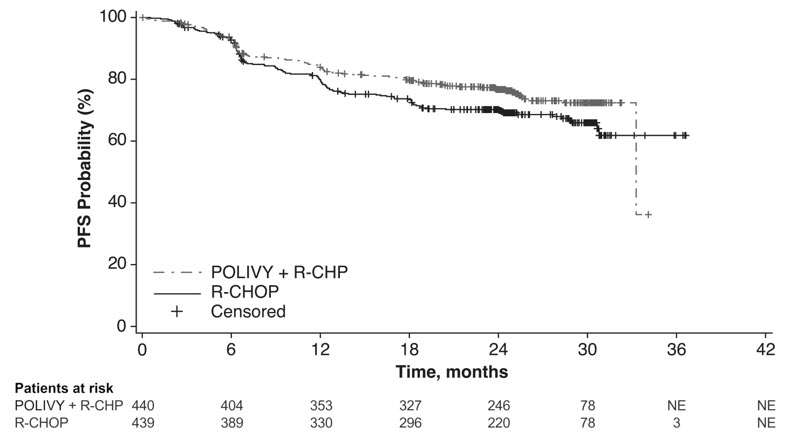
In a prespecified descriptive analysis of the largest lymphoma subgroup, DLBCL, NOS, the PFS HR was 0.75 (95% CI: 0.57, 0.99). In patients with HGBL, the PFS HR was 0.48 (95% CI: 0.21, 1.08). There were insufficient data to evaluate efficacy in other large B-cell lymphomas.
With an estimated median follow-up of 3.3 years, the prespecified final analysis of overall survival (OS) showed no statistically significant difference, with a HR of 0.94 (95% CI: 0.67, 1.33). In a descriptive analysis, the OS HR in patients with DLBCL, NOS was 1.02 (95% CI: 0.70, 1.49). The OS HR in patients with HGBL was 0.42 (95% CI: 0.15, 1.19).
Close14.2 Relapsed or Refractory DLBCL, NOS
GO29365
The efficacy of POLIVY was evaluated in Study GO29365 (NCT02257567), an open-label, multicenter clinical trial that included a cohort of 80 patients with relapsed or refractory DLBCL after at least one prior regimen. Patients were randomized 1:1 to receive either POLIVY in combination with bendamustine and a rituximab product (BR) or BR alone for six 21-day cycles. Randomization was stratified by duration of response (DOR) to last therapy. Eligible patients were not candidates for autologous HSCT at study entry. The study excluded patients with Grade 2 or higher peripheral neuropathy, prior allogeneic HSCT, active central nervous system lymphoma, or transformed lymphoma.
Following premedication with an antihistamine and antipyretic, POLIVY was given by intravenous infusion at 1.8 mg/kg on Day 2 of Cycle 1 and on Day 1 of Cycles 2–6. Bendamustine was administered at 90 mg/m2 intravenously daily on Days 2 and 3 of Cycle 1 and on Days 1 and 2 of Cycles 2–6. A rituximab product was administered at a dose of 375 mg/m2 intravenously on Day 1 of Cycles 1–6. The cycle length was 21 days.
Of the 80 patients randomized to receive POLIVY plus BR (n = 40) or BR alone (n = 40), the median age was 69 years (range: 30–86 years), 66% were male, and 71% were White. Most patients (98%) had DLBCL not otherwise specified. The primary reasons patients were not candidates for HSCT included age (40%), insufficient response to salvage therapy (26%), and prior transplant failure (20%). The median number of prior therapies was 2 (range: 1–7), with 29% receiving one prior therapy, 25% receiving 2 prior therapies, and 46% receiving 3 or more prior therapies. Eighty percent of patients had refractory disease to last therapy.
In the POLIVY plus BR arm, patients received a median of 5 cycles, with 49% receiving 6 cycles. In the BR arm, patients received a median of 3 cycles, with 23% receiving 6 cycles.
Efficacy was based on complete response (CR) rate at the end of treatment and DOR, as determined by an independent review committee (IRC). Other efficacy measures included IRC-assessed best overall response.
Response rates are summarized in Table 12.
Table 12 Response Rates in Patients with Relapsed or Refractory DLBCL Response per IRC, n (%)* POLIVY + BR
n = 40BR
n = 40CR=complete response; PR=partial remission. - *
- PET-CT based response per modified Lugano 2014 criteria. Bone marrow confirmation of PET-CT CR was required. PET-CT PR required meeting both PET criteria and CT criteria for PR.
- †
- End of treatment was defined as 6–8 weeks after Day 1 of Cycle 6 or last study treatment.
- ‡
- Miettinen-Nurminen method.
- §
- PET-CT results were prioritized over CT results.
Objective Response at End of Treatment†
(95% CI)18 (45)
(29, 62)7 (18)
(7, 33)CR
(95% CI)16 (40)
(25, 57)7 (18)
(7, 33)Difference in CR rates, % (95% CI)‡ 22 (3, 41) Best Overall Response of CR or PR§
(95% CI)25 (63)
(46, 77)10 (25)
(13, 41)Best Response of CR
(95% CI)20 (50)
(34, 66)9 (23)
(11, 38)In the POLIVY plus BR arm, of the 25 patients who achieved a partial or complete response, 16 (64%) had a DOR of at least 6 months, and 12 (48%) had a DOR of at least 12 months. In the BR arm, of the 10 patients who achieved a partial or complete response, 3 (30%) had a DOR lasting at least 6 months, and 2 (20%) had a DOR lasting at least 12 months.
-
15 REFERENCES"OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - POLIVY (polatuzumab vedotin-piiq) for injection is a preservative-free, white to grayish-white lyophilized powder, which has a cake-like appearance. POLIVY is supplied ...
How Supplied
POLIVY (polatuzumab vedotin-piiq) for injection is a preservative-free, white to grayish-white lyophilized powder, which has a cake-like appearance. POLIVY is supplied as:
Carton Contents NDC One 30 mg single-dose vial NDC 50242-103-01 One 140 mg single-dose vial NDC 50242-105-01 CloseStorage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do not use beyond the expiration date shown on the carton. Do not freeze. Do not shake.
POLIVY is a hazardous drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATIONPeripheral Neuropathy - Advise patients that POLIVY can cause peripheral neuropathy. Advise patients to report to their healthcare provider any numbness or tingling of the hands or feet or any ...
Peripheral Neuropathy
Advise patients that POLIVY can cause peripheral neuropathy. Advise patients to report to their healthcare provider any numbness or tingling of the hands or feet or any muscle weakness [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider if they experience signs and symptoms of infusion reactions, including fever, chills, rash, or breathing problems, within 24 hours of infusion [see Warnings and Precautions (5.2)].
Myelosuppression
Advise patients to report signs or symptoms of bleeding or infection immediately. Advise patients of the need for periodic monitoring of blood counts [see Warnings and Precautions (5.3)].
Infections
Advise patients to contact their healthcare provider if a fever of 38°C (100.4°F) or greater or other evidence of potential infection such as chills, cough, or pain on urination develops. Advise patients of the need for periodic monitoring of blood counts [see Warnings and Precautions (5.4)].
Progressive Multifocal Leukoencephalopathy
Advise patients to seek immediate medical attention for new or changes in neurological symptoms such as confusion, dizziness, or loss of balance; difficulty talking or walking; or changes in vision [see Warnings and Precautions (5.5)].
Tumor Lysis Syndrome
Advise patients to seek immediate medical attention for symptoms of tumor lysis syndrome such as nausea, vomiting, diarrhea, and lethargy [see Warnings and Precautions (5.6)].
Hepatotoxicity
Advise patients to report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with POLIVY [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential, and males with female partners of reproductive potential, to use effective contraception during treatment with POLIVY and for 3 months and 5 months after the last dose, respectively [see Use in Specific Populations (8.3)].
CloseLactation
Advise women not to breastfeed while receiving POLIVY and for 2 months after the last dose [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTIONPOLIVY® [polatuzumab vedotin-piiq] Manufactured by: Genentech, Inc. A Member of the Roche Group - 1 DNA Way - South San Francisco, CA 94080-4990 - POLIVY is a registered trademark of Genentech, Inc ...
POLIVY® [polatuzumab vedotin-piiq]
Manufactured by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990POLIVY is a registered trademark of Genentech, Inc.
© 2023 Genentech, Inc.U.S. License No. 1048
Close -
SPL UNCLASSIFIED SECTIONRepresentative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
PRINCIPAL DISPLAY PANEL - 140 mg Vial CartonNDC 50242-105-01 - Polivy® (polatuzumab vedotin-piiq) For Injection - 140 mg per vial - For Intravenous Infusion Only. Reconstitute and Dilute - prior to administration. Single-Dose Vial. Discard ...
NDC 50242-105-01
Polivy®
(polatuzumab vedotin-piiq)
For Injection140 mg per vial
For Intravenous Infusion Only.
Reconstitute and Dilute
prior to administration.
Single-Dose Vial.
Discard unused portion.CAUTION: Hazardous Agent
Rx only
1 vial
Genentech11007928
Close
-
PRINCIPAL DISPLAY PANEL - 30 mg Vial CartonNDC 50242-103-01 - Polivy® (polatuzumab vedotin-piiq) For Injection - 30 mg per vial - For Intravenous Infusion Only. Reconstitute and Dilute - prior to administration. Single-Dose Vial. Discard ...
NDC 50242-103-01
Polivy®
(polatuzumab vedotin-piiq)
For Injection30 mg per vial
For Intravenous Infusion Only.
Reconstitute and Dilute
prior to administration.
Single-Dose Vial.
Discard unused portion.CAUTION: Hazardous Agent
Rx only
1 vial
Genentech11008718
Close
-
INGREDIENTS AND APPEARANCEProduct Information
POLIVY polatuzumab vedotin injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-105 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLATUZUMAB VEDOTIN (UNII: KG6VO684Z6) (POLATUZUMAB VEDOTIN - UNII:KG6VO684Z6) POLATUZUMAB VEDOTIN 140 mg in 7.52 mL Inactive Ingredients Ingredient Name Strength SUCCINIC ACID (UNII: AB6MNQ6J6L) 8.27 mg in 7.52 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) 3.8 mg in 7.52 mL SUCROSE (UNII: C151H8M554) 288 mg in 7.52 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 8.4 mg in 7.52 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-105-01 1 in 1 CARTON 06/10/2019 1 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761121 06/10/2019 POLIVY polatuzumab vedotin injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-103 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLATUZUMAB VEDOTIN (UNII: KG6VO684Z6) (POLATUZUMAB VEDOTIN - UNII:KG6VO684Z6) POLATUZUMAB VEDOTIN 30 mg in 1.88 mL Inactive Ingredients Ingredient Name Strength SUCCINIC ACID (UNII: AB6MNQ6J6L) SODIUM HYDROXIDE (UNII: 55X04QC32I) SUCROSE (UNII: C151H8M554) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-103-01 1 in 1 CARTON 09/18/2020 1 6 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761121 06/10/2019 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 ANALYSIS(50242-103, 50242-105) , API MANUFACTURE(50242-103, 50242-105) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 146373191 ANALYSIS(50242-103, 50242-105) , API MANUFACTURE(50242-103, 50242-105) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd 485244961 MANUFACTURE(50242-103, 50242-105) , ANALYSIS(50242-103, 50242-105) , LABEL(50242-103, 50242-105) , PACK(50242-103, 50242-105) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 833220176 PACK(50242-105) , LABEL(50242-105) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 315028860 PACK(50242-103, 50242-105) , LABEL(50242-103, 50242-105) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 323105205 ANALYSIS(50242-103, 50242-105)
CloseEstablishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche AG 482242971 API MANUFACTURE(50242-103, 50242-105) , ANALYSIS(50242-103, 50242-105)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
Number of versions: 10
RxNorm
POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2174095 | polatuzumab vedotin-piiq 140 MG Injection | PSN |
| 2 | 2174095 | polatuzumab vedotin-piiq 140 MG Injection | SCD |
| 3 | 2174100 | Polivy 140 MG Injection | PSN |
| 4 | 2174100 | polatuzumab vedotin-piiq 140 MG Injection [Polivy] | SBD |
| 5 | 2174100 | Polivy 140 MG Injection | SY |
| 6 | 2398131 | olatuzumab vedotin-piiq 30 MG Injection | PSN |
| 7 | 2398131 | polatuzumab vedotin-piiq 30 MG Injection | SCD |
| 8 | 2398133 | Polivy 30 MG Injection | PSN |
| 9 | 2398133 | polatuzumab vedotin-piiq 30 MG Injection [Polivy] | SBD |
| 10 | 2398133 | Polivy 30 MG Injection | SY |
Get Label RSS Feed for this Drug
POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=20a16ab2-f338-4abb-9dcd-254bd949a2bc
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50242-103-01 |
| 2 | 50242-105-01 |


