Label: ANKTIVA- nogapendekin alfa inbakicept-pmln solution
- NDC Code(s): 81481-803-01
- Packager: Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ANKTIVA safely and effectively. See full prescribing information for ANKTIVA.
ANKTIVA® (nogapendekin alfa inbakicept-pmln) solution, for intravesical use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
ANKTIVA is an interleukin-15 (IL-15) receptor agonist indicated with Bacillus Calmette-Guérin (BCG) for the treatment of adult patients with BCG-unresponsive nonmuscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors. ( 1)
DOSAGE AND ADMINISTRATION
For Intravesical Use Only
- For induction: 400 mcg administered intravesically with BCG once a week for 6 weeks. A second induction course may be administered if complete response is not achieved at month 3. (
2.1)
- For maintenance: 400 mcg administered intravesically with BCG once a week for 3 weeks at months 4, 7, 10, 13 and 19. For patients with an ongoing complete response at month 25 and later, additional maintenance instillations with BCG may be administered once a week for 3 weeks at months 25, 31, and 37. (
2.1)
- Instill intravesically only after dilution. Total time from vial puncture to the completion of the intravesical instillation should not exceed 2 hours. (
2.2)
See full Prescribing Information for dilution and administration instructions.
DOSAGE FORMS AND STRENGTHS
400 mcg/0.4 mL, clear to slightly opalescent and colorless to slightly yellow solution in single-dose vials for intravesical instillation after dilution. ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
- Delaying cystectomy can lead to the development of metastatic bladder cancer, which can be lethal. ( 5.1)
ADVERSE REACTIONS
The most common (≥15%) adverse reactions, including laboratory test abnormalities, are increased creatinine, dysuria, hematuria, urinary frequency, micturition urgency, urinary tract infection, increased potassium, musculoskeletal pain, chills and pyrexia. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc. at toll-free phone 877-265-8482 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
- For induction: 400 mcg administered intravesically with BCG once a week for 6 weeks. A second induction course may be administered if complete response is not achieved at month 3. (
2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Metastatic Bladder Cancer with Delayed Cystectomy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
For Intravesical Use Only. Do NOT administer by subcutaneous or intravenous or intramuscular routes.
- For induction: ANKTIVA is recommended at a dose of 400 mcg administered intravesically with BCG once a week for 6 weeks. A second induction course may be administered if complete response is not achieved at month 3.
- For maintenance: After BCG and ANKTIVA induction therapy, ANKTIVA is recommended at a dose of 400 mcg administered intravesically with BCG once a week for 3 weeks at months 4, 7, 10, 13 and 19 (for a total of 15 doses). For patients with an ongoing complete response at month 25 and later, maintenance instillations with BCG may be administered once a week for 3 weeks at months 25, 31, and 37 for a maximum of 9 additional instillations.
The recommended duration of treatment is until disease persistence after second induction, disease recurrence or progression, unacceptable toxicity, or a maximum of 37 months.
2.2 Preparation and Administration
Preparation of Agent
See BCG Prescribing Information for information on preparation and handling of BCG.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution is clear to slightly opalescent and colorless to slightly yellow. Discard the vial if visible particles are observed.
Draw 0.4 mL of ANKTIVA into a small syringe and using aseptic technique add to the saline containing the BCG suspension that has been prepared following the instructions provided in the Prescribing Information for BCG. Mix the suspension gently. Using a 60-mL syringe connected to an appropriate size needle, withdraw the ANKTIVA BCG mixture to a final volume of 50 mL.
If the admixture of ANKTIVA in combination with BCG is not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) and use within 2 hours. Unused solution of admixture should be discarded after 2 hours.
Treatment
The admixture of ANKTIVA in combination with BCG is instilled into the bladder via a catheter. After instillation is complete, the catheter is removed. The ANKTIVA in combination with BCG admixture is retained in the bladder for 2 hours and then voided. Patients unable to retain the suspension for 2 hours should be allowed to void sooner, if necessary. Do not repeat the dose if the patient voids before 2 hours.See BCG Prescribing Information for information on retention in the bladder and patient positioning during bladder instillation.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Metastatic Bladder Cancer with Delayed Cystectomy
Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. The risk of developing muscle invasive or metastatic bladder cancer increases the longer cystectomy is delayed in the presence of persisting CIS.
Of the 77 evaluable patients with BCG-unresponsive CIS treated with ANKTIVA with BCG in QUILT-3.032, 10% (n = 8) progressed to muscle invasive (T2 or greater) bladder cancer, including 7 during the treatment period. Three patients had progression determined at the time of cystectomy. The median time between determination of persistent or recurrent CIS and progression to muscle-invasive disease was 107 days (range: 0 – 210).
If patients with CIS do not have a complete response to treatment after a second induction course of ANKTIVA with BCG, reconsider cystectomy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ANKTIVA with BCG was evaluated in Cohort A of QUILT-3.032, a single-arm, multicenter clinical study in 88 patients with BCG-unresponsive high-grade NMIBC with CIS with or without Ta/T1 papillary disease [see Clinical Studies ( 14)]. Patients received 400 mcg ANKTIVA with BCG weekly for 6 consecutive weeks during induction and then once a week for every 3 weeks at 4, 7, 10, 13, and 19 months for patients with no or low grade disease. Patients with persistent CIS or high grade Ta at 3 months were eligible to receive a second induction. Patients with ongoing CR at 25 months were eligible to receive additional instillations once a week every 3 weeks at months 25, 31, and 37. The median number of doses of ANKTIVA with BCG administered to patients was 12 (range 2 – 30) doses. The median duration of exposure to ANKTIVA with BCG was 7.1 months (range: 0.26 to 36.3 months).
Serious adverse reactions occurred in 16% of patients receiving ANKTIVA with BCG. Serious adverse reactions that occurred in ≥2% of patients who received ANKTIVA with BCG included hematuria (3.4%). A fatal adverse reaction of cardiac arrest occurred in 1 (1.1%) patient receiving ANKTIVA with BCG.
Permanent discontinuation of ANKTIVA with BCG due to adverse reactions occurred in 7% of patients. Adverse reactions (>2%) resulting in permanent discontinuation of ANKTIVA with BCG included musculoskeletal pain (2.3%).
Dosage interruptions due to adverse reactions occurred in 34% of patients receiving ANKTIVA with BCG. Adverse reactions (≥5%) that resulted in interruption of ANKTIVA with BCG were urinary tract infection (10%), dysuria (8%), hematuria (6%), and bladder irritation (6%).
Dosage reductions due to adverse reactions were not permitted for ANKTIVA; however, dose reduction of BCG was allowed for adverse reactions and occurred in 3.4% of patients including (>1%) urinary tract infection (2.3%), hematuria (1.1%), urinary frequency (1.1%), and bladder irritation (1.1%).
The most common (≥15%) adverse reactions, including laboratory test abnormalities, were increased creatinine, dysuria, hematuria, urinary frequency, micturition urgency, urinary tract infection, increased potassium, musculoskeletal pain, chills and pyrexia.
Table 1 summarizes the adverse reactions in Cohort A of QUILT-3.032.
Table 1: Adverse Reactions Occurring in ≥15% of Patients in Cohort A in QUILT-3.032 1Includes other related terms Adverse Reaction ANKTIVA with BCG
(n=88)All Grades
%Grades 3 or 4
%Dysuria 32 0 Hematuria 1 32 3.4 Urinary Frequency 27 0 Micturition Urgency 1 25 0 Urinary Tract Infection 1 24 2.3 Musculoskeletal Pain 1 17 2.3 Chills 15 0 Pyrexia 15 0 Clinically relevant adverse reactions in <15% of patients who received ANKTIVA with BCG included fatigue (14%), nausea (14%), bladder irritation (11%), diarrhea (9%), and nocturia (7%).
Table 2 summarizes the laboratory test abnormalities occuring in ≥15% of patients in QUILT-3.032.
Table 2: Select Laboratory Test Abnormalities (≥15%) That Worsened From Baseline in Patients in Cohort A of QUILT-3.032 1The denominator used to calculate the rates was 88 based on the number of patients with a baseline value and at least one post-treatment value. Laboratory Abnormality ANKTIVA with BCG 1
(n=88)All Grades
%Grades 3 or 4
%Increased Creatinine 76 0 Increased Potassium 18 2 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Systemic exposure of nogapendekin alfa inbakicept-pmln following intravesical administration of the approved dosage of ANKTIVA was below the limit of quantitation [see Clinical Pharmacology 12.3)] .Based on its mechanism of action, ANKTIVA may cause fetal harm when administered to a pregnant woman if systemic exposure occurs [see Clinical Pharmacology ( 12.1)] . There are no available data on ANKTIVA use in pregnant women to inform a drug-associated risk. Animal reproductive and developmental toxicity studies have not been conducted with nogapendekin alfa inbakicept-pmln. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of nogapendekin alfa inbakicept-pmln in human milk, or the effects on the breastfed child, or on milk production. Systemic exposure of nogapendekin alfa inbakicept-pmln in patients receiving intravesical administration of the approved dosage of ANKTIVA was below the limit of quantitation [see Clinical Pharmacology ( 12.3)] , indicating any amount in the milk will be low. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ANKTIVA and any potential adverse effects on the breastfed child from ANKTIVA or from the underlying maternal condition.8.3 Females and Males of Reproductive Potential
Based on its mechanism of action, ANKTIVA may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)] .
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating treatment with ANKTIVA.Contraception
Advise females of reproductive potential to use effective contraception during treatment with ANKTIVA and for 1 week after the last dose.8.4 Pediatric Use
Safety and effectiveness of ANKTIVA in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients in clinical studies of ANKTIVA for BCG-unresponsive NMIBC, 84% were 65 years of age or older and 40% were 75 years or older. Clinical studies of ANKTIVA did not include sufficient numbers of younger adult patients to determine if patients 65 years of age and older respond differently than younger adult patients.
-
11 DESCRIPTION
Nogapendekin alfa inbakicept-pmln is an interleukin-15 (IL-15) receptor agonist. It is a soluble complex consisting of (a) nogapendekin alfa (a human IL-15N72D variant, 114 amino acids) bound to (b) inbakicept [a dimeric human IL-15Rα sushi domain (65 amino acids)/human IgG1 Fc fusion protein (232 amino acids)]. Each fully assembled nogapendekin alfa inbakicept-pmln complex consists of a single inbakicept and two nogapendekin alfa components. Each IL-15N72D component is bound to one of the IL-15Rα sushi domains.
The recombinant protein complex is produced by a recombinant cell line that was created by transfecting a Chinese hamster ovary (CHO-K1) cell line with plasmids carrying genes for the IL-15N72D and IL-15RαSu/IgG1 Fc proteins. Purification of the product is achieved by conventional chromatography. The molecular weight of the deglycosylated nogapendekin alfa inbakicept-pmln complex is 92,106.5 Da. The molecular weights of the individual deglycosylated sub-units are 66565.6 Da (dimer) and 12,770.45 Da for the IL-15RαSu/IgG1 Fc domain and the IL-15N72D domain, respectively.
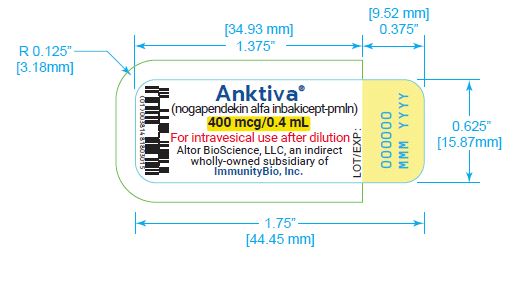
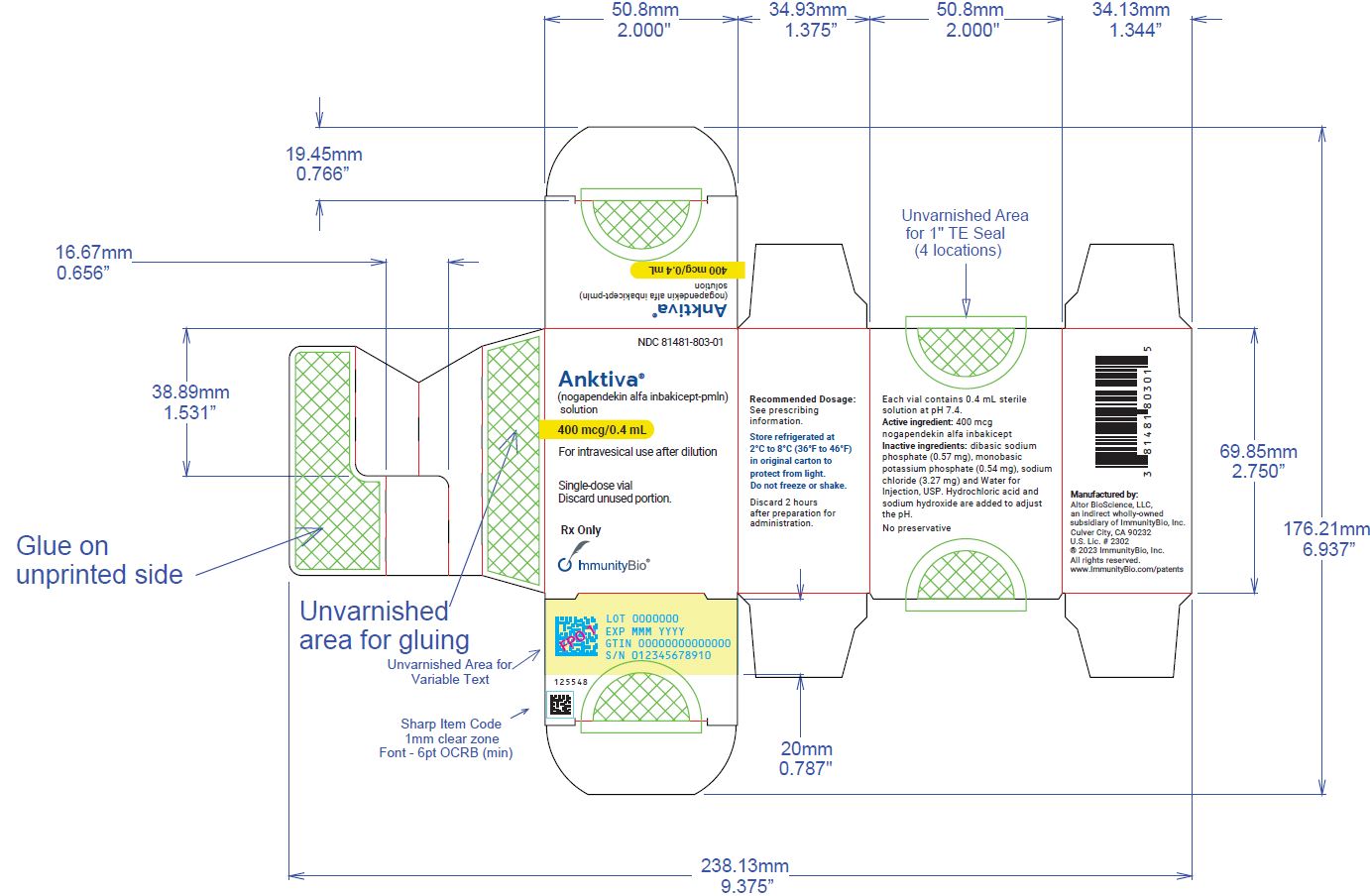
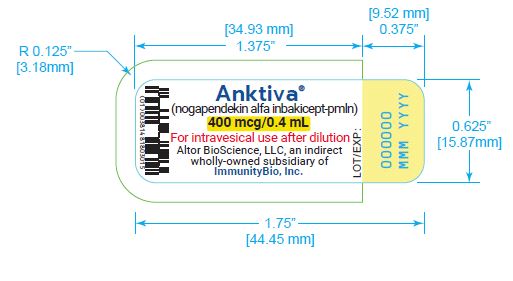
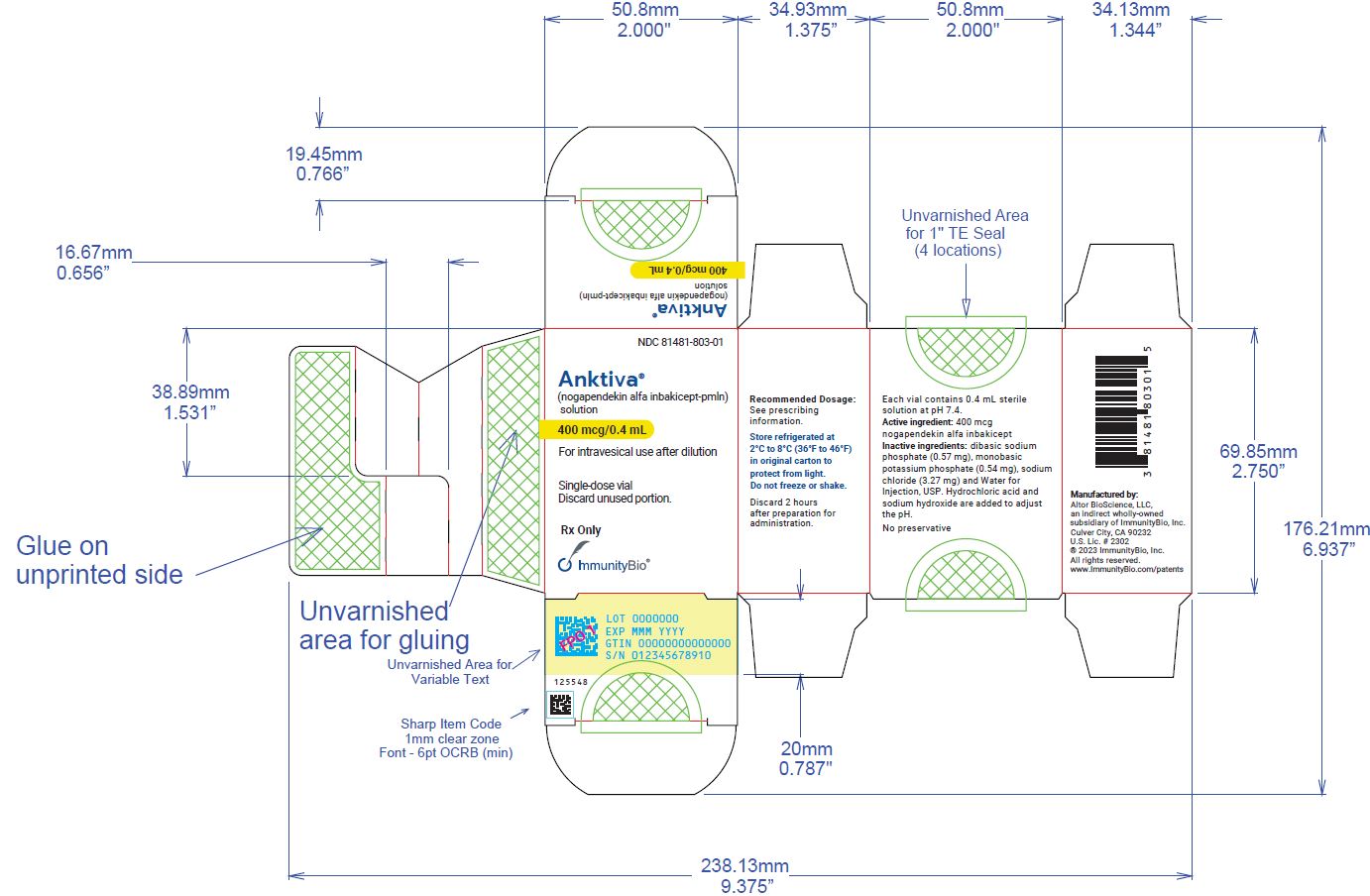
ANKTIVA (nogapendekin alfa inbakicept-pmln) solution is a clear to slightly opalescent, colorless to slightly yellow solution provided in a single-dose vial containing 400 mcg in 0.4 mL for intravesical administration upon dilution [see Dosage and Administration ( 2)] . Each vial also contains dibasic sodium phosphate (0.57 mg), monobasic potassium phosphate (0.54 mg), sodium chloride (3.27 mg) and Water for Injection, USP. Hydrochloric acid and sodium hydroxide is added to adjust the pH to 7.4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nogapendekin alfa inbakicept-pmln is an IL-15 receptor agonist. IL-15 signals through a heterotrimeric receptor that is composed of the common gamma chain (γc) subunit, the beta chain (βc) subunit, and the IL-15-specific alpha subunit, IL-15 receptor α. IL-15 is trans-presented by the IL-15 receptor α to the shared IL-2/IL-15 receptor (βc and γc) on the surface of CD4 +and CD8 +T cells and NK cells.
Binding of nogapendekin alfa inbakicept-pmln to its receptor results in proliferation and activation of NK, CD8 +, and memory T cells without proliferation of immuno-suppressive Treg cells. In vivo, intravesicular nogapendekin alfa inbakicept-pmln alone or in combination with BCG showed anti-tumor activity when compared to BCG alone, in a carcinogen-induced model of bladder cancer in immunocompetent rats.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of nogapendekin alfa inbakicept-pmln have not been fully characterized.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of ANKTIVA was evaluated in QUILT-3.032 (NCT03022825), a single-arm, multicenter trial in 77 adults with BCG-unresponsive, high-risk, NMIBC with CIS with or without Ta/T1 papillary disease following transurethral resection.
BCG unresponsive high-risk NMIBC CIS was defined as persistent or recurrent CIS alone or with Ta/T1 disease within 12 months of completion of adequate BCG therapy. Adequate BCG therapy was defined as administration of at least 5 of 6 doses of an initial induction course plus either of at least 2 of 3 doses of maintenance therapy or at least 2 of 6 doses of a second induction course. Prior to treatment, all patients with Ta or T1 disease had undergone transurethral resection of bladder tumor (TURBT) to remove all resectable disease. Residual CIS not amenable to complete resection, fulguration, or cauterization was permitted. The trial excluded patients with history of or evidence of muscle invasive (i.e., T2, T3, T4), locally advanced, metastatic, and/or extra-vesical (i.e., urethra, ureter, or renal pelvis) bladder cancer.
Patients received 400 mcg ANKTIVA with BCG weekly for 6 consecutive weeks during the induction treatment period and then once a week every 3 weeks at 4, 7, 10, 13, and 19 months for patients with no or low grade disease. Patients with persistent CIS or high grade Ta disease at 3 months were eligible to receive a second induction course. Patients with ongoing CR at 25 months were eligible to receive additional instillations once a week every 3 weeks at months 25, 31, and 37. Assessment of tumor status was performed every 3 months for up to two years. Assessment for ongoing response beyond month 24 was per local community standards. Random or cystoscopy directed biopsies were required within the first 6 months after treatment initiation. The major efficacy outcome measures were complete response (CR) at any time (as defined by negative results for cystoscopy [with TURBT/biopsies as applicable] and urine cytology) and duration of response.
The median age of patients was 73 years (range, 50–91 years); 86% were male; race was White (90%), Black (6%), Asian (1%), American Indian or Alaska Native (1%), or Unknown (1%); and patients had baseline ECOG performance status of 0 (83%) or 1 (17%).
Tumor characteristics at study entry were CIS without Ta/T1 papillary disease (69%), CIS with Ta papillary disease (21%) or CIS with T1 +/- Ta papillary disease (10%). Baseline high-risk NMIBC disease status was 43% refractory and 57% relapsed. The median number of prior BCG doses received was 12 doses (range: 8–45 doses); 13% received partial-dose prior BCG. Baseline cystoscopy imaging modality was white light (57%), blue light or narrow band imaging (40%), and unknown (3%).
Efficacy results are summarized in Table 3. Thirty-one percent (n=24) of patients received a second induction course.
Table 3: Efficacy Results in QUILT-3.032 + Denotes ongoing response
aBased on 48 patients that achieved a complete response at any time; reflects period from the time complete response was achieved.ANKTIVA with BCG
(n=77)Complete Response Rate (95% CI) 62% (51, 73) Duration of Response a Range in months 0.0, 47.0+ % (n) with duration ≥ 12 months 58% (28) % (n) with duration ≥ 24 months 40% (19) -
16 HOW SUPPLIED/STORAGE AND HANDLING
ANKTIVA (nogapendekin alfa inbakicept-pmln) is clear to slightly opalescent and colorless to slightly yellow solution available in:
Carton containing 400 mcg/0.4 mL, single-dose vial (NDC 81481-803-01).
Store vials under refrigeration at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Risk of Metastatic Bladder Cancer with Delayed Cystectomy
- Inform patients that delaying cystectomy could lead to development of metastatic bladder cancer. Discuss the risk of metastatic bladder cancer and that the risk increases the longer cystectomy is delayed in the presence of persisting CIS [see Warnings and Precautions ( 5.1)].
Local Adverse Reactions Before and During Treatment
- Inform patients that irritable bladder symptoms may occur during instillation, and retention of ANKTIVA, and following voiding [see Adverse Reactions ( 6.1)].
- Inform patients that for the first 24 hours following administration, red-tinged urine may occur.
- Advise patients to immediately report prolonged irritable bladder symptoms or prolonged passage of red-colored urine to their healthcare provider.
- Instruct patients to maintain adequate hydration following ANKTIVA treatment.
- Inform patients that the ANKTIVA in combination with BCG admixture should be retained in the bladder for 2 hours and then voided.
- Advise patients that if unable to retain the suspension for 2 hours they can void sooner if necessary [see Dosage and Administration ( 2.2)]
- Instruct patients to void while seated to avoid splashing of urine.
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations ( 8.1)] .
- Advise females of reproductive potential to use effective contraception during treatment with ANKTIVA and for 1 week after the last dose [see Use in Specific Populations ( 8.3)] .
See the BCG prescribing information, Information for Patients for additional patient counseling information.
Manufactured for: Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc., Culver City, CA 90232 Manufactured by AGC Biologics, 21511 23 rdDr. SE, Bothell, WA 98021
U.S. license number 2302 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANKTIVA
nogapendekin alfa inbakicept-pmln solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81481-803 Route of Administration INTRAVESICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOGAPENDEKIN ALFA INBAKICEPT-PMLN (UNII: 7TK323DLA0) (NOGAPENDEKIN ALFA INBAKICEPT-PMLN - UNII:7TK323DLA0) NOGAPENDEKIN ALFA INBAKICEPT-PMLN 400 ug in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 0.57 mg in 0.4 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.27 mg in 0.4 mL MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) 0.54 mg in 0.4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81481-803-01 1 in 1 BOX 05/06/2024 1 0.4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761336 05/06/2024 Labeler - Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc. (138254896) Registrant - Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc. (138254896) Establishment Name Address ID/FEI Business Operations Ajinomoto Bio-Pharma Services 023050730 manufacture(81481-803)