Label: XOLREMDI- mavorixafor capsule, gelatin coated
- NDC Code(s): 83296-100-12, 83296-100-60
- Packager: X4 Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XOLREMDI™ safely and effectively. See full prescribing information for XOLREMDI™.
XOLREMDI™ (mavorixafor) capsules, for oral use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
XOLREMDI is a CXC chemokine receptor 4 antagonist indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lymphocytes. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 100 mg mavorixafor. ( 3)
WARNINGS AND PRECAUTIONS
- Embryo-fetal toxicity: Expected to cause fetal harm. Advise women of reproductive potential to use effective contraception. ( 5.1, 8.1, 8.3)
- QTc Interval Prolongation: : Correct any modifiable risk factors, assess QTc at baseline and monitor QTc during treatment as clinically indicated. XOLREMDI dose reduction or discontinuation may be required due to drug-drug interactions. ( 5.2)
ADVERSE REACTIONS
The most common adverse reactions (≥10% and at a frequency higher than placebo) were: thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact X4 Pharmaceuticals, Inc. at 1-866-MED-X4MI (1-866-633-9464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong CYP3A4 inhibitors: Reduce XOLREMDI daily dosage. ( 2.2, 7.1)
- P-gp inhibitors or moderate CYP3A4 inhibitors: Monitor more frequently for XOLREMDI adverse reactions and reduce XOLREMDI daily dosage if necessary. ( 7.1)
- Strong CYP3A4 Inducers: Avoid concomitant use. ( 7.1)
- CYP3A4 or P-gp substrates: Monitor more frequently for substrate adverse reactions unless otherwise recommended. ( 7.2)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Strong CYP3A4 inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 QTc Interval Prolongation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XOLREMDI
7.2 Effect of XOLREMDI on Other Drugs
7.3 Drugs that Prolong the QTc Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of XOLREMDI is:
- Weight more than 50 kg: 400 mg orally once daily on an empty stomach after an overnight fast, and at least 30 minutes before food.
- Weight less than or equal to 50 kg: 300 mg orally once daily on an empty stomach after an overnight fast, and at least 30 minutes before food.
Swallow the capsules whole. Do not open, break, or chew capsules.
If a dose of XOLREMDI is missed, the next dose should be taken as scheduled. Do not take more than one XOLREMDI dose each day.
2.2 Dosage Modifications for Strong CYP3A4 inhibitors
Reduce daily dosage of XOLREMDI to 200 mg when used concomitantly with strong CYP3A4 inhibitors [see Drug Interactions (7.1)] .
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Use of XOLREMDI is contraindicated with drugs that are highly dependent on CYP2D6 for clearance [see Drug Interactions (7.2)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Based on its mechanism of action, XOLREMDI is expected to cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.2)] . Animal models link reductions in CXCR4/SDF-1 signaling to adverse outcomes in mammalian embryo-fetal development and to abnormal placental development.
Verify the pregnancy status of female patients of reproductive potential prior to starting XOLREMDI. Advise females of reproductive potential to use an effective method of contraception during treatment with XOLREMDI and for three weeks after the final dose [see Use in Specific Populations (8.1and 8.3)] .
5.2 QTc Interval Prolongation
XOLREMDI causes concentration-dependent QTc interval prolongation. QT interval prolongation may occur when XOLREMDI is taken with concomitant medications that increase XOLREMDI exposure and/or drug products with a known potential to prolong QT. Correct any modifiable risk factors for QTc prolongation (e.g., hypokalemia), assess QTc at baseline and monitor QTc during treatment as clinically indicated in patients with risk factors for QTc prolongation such as those receiving concomitant medications that increase XOLREMDI exposure and drug products with a known potential to prolong the QTc interval. A dose reduction in XOLREMDI or discontinuation of XOLREMDI may be required [see Drug Interactions (7.1and 7.3)and Clinical Pharmacology (12.2)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QTc Interval Prolongation [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of XOLREMDI was evaluated in Study 1, a randomized placebo-controlled trial of 31 adult and pediatric patients 12 years and older with WHIM syndrome [see Clinical Studies (14)] . Patients received XOLREMDI 400 mg or 200 mg, based on age and body weight (N=14) or placebo (N=17). One patient received the 200 mg dose, and 13 patients received the 400 mg dose. Note that the 200 mg XOLREMDI daily dose is only recommended for use in patients receiving strong CYP3A4 inhibitors [see Dosage and Administration (2.1and 2.2)] . For all other patients, the recommended dosage is either 400 mg daily (if weighing more than 50 kg) or 300 mg daily (if weighing up to 50 kg), unless dose reductions are needed due to concomitant use with moderate CYP3A4 inhibitors or P-gp inhibitors [see Drug Interactions (7.1)] .
The data below are based on the 52-week, placebo-controlled portion of the study. Twelve patients received XOLREMDI for at least 6 months, and 10 patients received XOLREMDI for at least one year.
Table 1 summarizes the most common adverse reactions (>10%) in Study 1, which were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
Table 1: Adverse Reactions in ≥10% Patients with WHIM Syndrome Receiving XOLREMDI and More Frequently Reported than Placebo During Study 1 Number (N) and Percent (%) of Patients Adverse Reaction XOLREMDI
(N=14)Placebo
(N=17)Thrombocytopenia 3 (21%) 0 Pityriasis 2 (14%) 0 Rash 2 (14%) 0 Rhinitis 2 (14%) 0 Epistaxis 2 (14%) 1 (6%) Vomiting 2 (14%) 1 (6%) Dizziness 2 (14%) 1 (6%) Serious adverse reactions of thrombocytopenia occurred in 3 of the 14 patients who received XOLREMDI, two of which occurred in the setting of infection or febrile neutropenia.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XOLREMDI
Strong or Moderate CYP3A4 Inhibitors
Reduce XOLREMDI daily dosage when used concomitantly with a strong CYP3A4 inhibitor [see Dosage and Administration (2.2)] .
Monitor more frequently for XOLREMDI adverse reactions that may be associated with an increase in mavorixafor exposure when used concomitantly with moderate CYP3A4 inhibitor and reduce the XOLREMDI daily dosage by steps of 100 mg, if necessary, but not to a dose less than 200 mg.
Mavorixafor is a CYP3A4 substrate. Concomitant use with a strong CYP3A4 inhibitor increases mavorixafor maximal concentrations (C max) and area under the concentration-time curve (AUC) [see Clinical Pharmacology (12.3)] , which may increase the risk of XOLREMDI adverse reactions.
Strong CYP3A4 Inducers
Avoid concomitant use with a strong CYP3A4 inducer.
Mavorixafor is a CYP3A4 substrate. Concomitant use with a strong CYP3A4 inducer is predicted to decrease mavorixafor C maxand AUC based upon a mechanistic understanding of its elimination [see Clinical Pharmacology (12.3)] , which may reduce XOLREMDI's effectiveness.
P-gp Inhibitors
Monitor more frequently for XOLREMDI adverse reactions that may be associated with an increase in mavorixafor exposure when used concomitantly with P-gp inhibitors and reduce the XOLREMDI daily dosage by steps of 100 mg, if necessary, but not to a dose less than 200 mg.
Mavorixafor is a P-gp substrate. Concomitant use with a P-gp inhibitors increases mavorixafor C maxand AUC [see Clinical Pharmacology (12.3)] , which may increase the risk of XOLREMDI adverse reactions.
7.2 Effect of XOLREMDI on Other Drugs
CYP2D6 Substrates
The use of XOLREMDI with drugs that are another drug highly dependent on CYP2D6 for clearance is contraindicated [see Contraindications (4)] .
Mavorixafor is a CYP2D6 inhibitor. Mavorixafor increases exposure of CYP2D6 substrates [see Clinical Pharmacology (12.3)] , which may increase the risk of adverse reactions related to these substrates.
CYP3A4 Substrates
Monitor for CYP3A4 substrate related adverse reactions more frequently, unless otherwise recommended in the substrate's Prescribing Information, when XOLREMDI is used concomitantly with CYP3A4 substrates where minimal substrate concentration changes may lead to serious adverse reactions.
Mavorixafor is an inhibitor of CYP3A4. Mavorixafor may increase the C maxand AUC of CYP3A4 substrates [see Clinical Pharmacology (12.3)] , which may increase the risk of adverse reactions from the CYP3A4 substrate.
P-gp Substrates
Monitor for P-gp substrate related adverse reactions more frequently, unless otherwise recommended in the substrate Prescribing Information, when XOLREMDI is used concomitantly with P-gp substrates where minimal substrate concentration changes may lead to serious adverse reactions.
Digoxin: Measure serum digoxin concentrations before initiating concomitant use with XOLREMDI, and continue monitoring serum digoxin concentrations as recommended in the Prescribing Information for digoxin [see Clinical Pharmacology (12.3)] .
Mavorixafor is an inhibitor of P-gp. Mavorixafor may increase the C maxand AUC of P-gp substrates [see Clinical Pharmacology (12.3)] , which may increase the risk of adverse reactions from the P-gp substrate.
7.3 Drugs that Prolong the QTc Interval
Obtain an electrocardiogram when initiating, during concomitant use, and as clinically indicated in patients receiving concomitant medications with a known potential to prolong the QTc interval [see Warnings and Precautions (5.2)] .
XOLREMDI causes QTc interval prolongation [see Clinical Pharmacology (12.2)] . Concomitant use of XOLREMDI with other products that prolong the QTc interval may result in a greater increase in the QTc interval and adverse reactions associated with QTc interval prolongation, including torsade de pointes, other serious arrythmias, and sudden death [see Warnings and Precautions (5.2)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, XOLREMDI is expected to cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] . There are no available data on XOLREMDI use in pregnant women informing the risk of embryo-fetal developmental toxicities. Animal models link reductions in CXCR4/SDF-1 signaling to adverse outcomes in mammalian embryo-fetal development (see Data) . No definitive animal studies have been conducted to evaluate the effect of mavorixafor on reproduction and fetal development.
Advise pregnant women of the potential risk to the fetus and to use effective contraception [see Warnings and Precautions (5.1)and Use in Specific Populations (8.3)] .
The estimated background risk of major birth defects and miscarriages for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with mavorixafor to evaluate effects on reproduction and embryo-fetal development. CXCR4/SDF-1 signaling plays an important role in mammalian embryo-fetal and placental development. In mice, CXCR4-/- knockout is embryo lethal and causes multiple developmental toxicities, most notably in the hematopoietic, cardiovascular and nervous systems. CXCR4/SDF-1 levels have a key role in stimulating trophoblast proliferation and differentiation necessary for appropriate placental growth and function in humans [see Warnings and Precautions (5.1)].
8.2 Lactation
Risk Summary
There are no data on the presence of mavorixafor in human or animal milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose.
8.3 Females and Males of Reproductive Potential
XOLREMDI is expected to cause fetal harm when administered to pregnant women [see Warnings and Precautions (5.1)and Use in Specific Populations (8.1)] .
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating XOLREMDI [see Use in Specific Populations (8.1)] .
Contraception
Advise females of reproductive potential to use an effective form of contraception during treatment with XOLREMDI and for three weeks after the final dose [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of XOLREMDI in WHIM syndrome for increasing the number of circulating mature neutrophils and lymphocytes have been established in pediatric patients aged 12 years and older. Use of XOLREMDI for this indication is supported by evidence from an adequate and well-controlled study in adults and pediatric patients aged 12 years and older [see Clinical Pharmacology (12.3)and Clinical Studies (14)] .
The safety and effectiveness of XOLREMDI have not been established in pediatric patients younger than 12 years of age.
8.5 Geriatric Use
In clinical studies of XOLREMDI in patients with WHIM syndrome, 2 (5%) patients were aged 65 years and older, and no patients were aged 75 years and older. Clinical studies did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients.
8.6 Renal Impairment
XOLREMDI is not recommended in patients with severe renal impairment (creatinine clearance [CLcr] 15 to less than 30 mL/min) or end-stage renal disease (CLcr less than 15 mL/min). No dosage adjustment is recommended in patients with mild to moderate renal impairment (CLcr 30 to less than 90 mL/min).
No clinically significant differences in the pharmacokinetics of mavorixafor were observed in mild to moderate renal impairment (CLcr 30 to less than 90 mL/min). The pharmacokinetics of mavorixafor have not been studied in subjects with severe renal impairment or end-stage renal disease [see Clinical Pharmacology (12.3)] .
8.7 Hepatic Impairment
XOLREMDI is not recommended for use in patients with moderate to severe hepatic impairment. No dosage adjustment is recommended in patients with mild hepatic impairment.
The pharmacokinetics of mavorixafor have not been studied in subjects with moderate to severe hepatic impairment [ see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Mavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist [see Clinical Pharmacology (12.1)] .
The chemical name of the active ingredient, mavorixafor, is N1-(1 H-benzimidazol-2-ylmethyl)- N1-[(8 S)-5,6,7,8-tetrahydroquinolin-8-yl]butane-1,4-diamine. It has a molecular formula of C 21H 27N 5and a molecular weight of 349.48 g/mol. Mavorixafor is of the Sconfiguration and its structural formula is provided in Figure 1.
Figure 1: Structural Formula
Mavorixafor is optically active and is a white to pale yellow to light brown solid. Mavorixafor is hygroscopic above relative humidities of 70%.
Mavorixafor is freely soluble in methanol, 95% ethanol and n-octanol, soluble in toluene, sparingly soluble in DMSO and acetonitrile, and very slightly soluble in HPLC grade water according to the USP solubility criteria.
Mavorixafor is soluble in pH 1.2 to 5.5 aqueous buffers and in pH 6.0 aqueous buffer, sparingly soluble in pH 6.8 aqueous buffer and slightly soluble in pH 7.5 aqueous buffer, according to the USP solubility criteria.
XOLREMDI is a hard gelatin capsule for oral administration. Each capsule contains 100 mg of mavorixafor with the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, microcrystalline cellulose, sodium lauryl sulfate, and sodium stearyl fumarate. The hard gelatin capsule contains FD&C Blue #2, gelatin, and titanium dioxide. The Black Ink contains ammonium hydroxide 28%, ferrosoferric oxide/black iron oxide (E172), isopropyl alcohol, n-butyl alcohol, propylene glycol, and shellac glaze in ethanol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist that blocks the binding of the CXCR4 ligand, stromal-derived factor-1α (SDF-1α)/CXC Chemokine Ligand 12 (CXCL12). SDF-1/CXCR4 plays a role in trafficking and homing of leukocytes to and from the bone marrow compartment. Gain of function mutations in the CXCR4 receptor gene that occur in patients with WHIM syndrome lead to increased responsiveness to CXCL12 and retention of leukocytes in the bone marrow. Mavorixafor inhibits the response to CXCL12 in both wild‑type and for mutated CXCR4 variants associated with WHIM syndrome. Treatment with mavorixafor results in increased mobilization of leukocytes and lymphocytes from the bone marrow into peripheral circulation.
12.2 Pharmacodynamics
Absolute Neutrophil Count (ANC) and Absolute Lymphocyte Count (ALC)
ANC and ALC peaked at 4 hours after XOLREMDI dosing and returned towards baseline within 24 h after dosing. Over mavorixafor doses of 50 mg (0.125 times the maximum recommended dosage) to 400 mg once daily, higher mavorixafor exposure at steady state was associated with longer mean time (hours) above ANC threshold (TAT ANC) of 500 cells/µL and longer mean time (hours) above ALC threshold (TAT ALC) of 1,000 cells/µL over a 24-hour period.
Cardiac Electrophysiology
In a thorough QT (TQT) study, the maximum mean increase in the QTc interval was 15.6 ms (upper bound of the 90% confidence interval = 19.8 ms) after administration of XOLREMDI 800 mg (2 times the maximum recommended dose) in healthy volunteers [ see Warnings and Precautions (5.2)]. In concentration-QT analysis the increase in the QTc interval was concentration-dependent.
12.3 Pharmacokinetics
Mavorixafor pharmacokinetic parameters are presented as geometric mean (CV%) in adults with WHIM syndrome unless otherwise specified. Mavorixafor steady state C maxis 3304 (58.6%) ng/mL and the AUC from 0 to 24 hours (AUC 0-24h) is 13970 (58.4%) ng*h/mL following 400 mg once daily.
Mavorixafor demonstrates nonlinear pharmacokinetics with greater than dose-proportional increases in C maxand AUC 0-24over a dose range of 50 mg (0.125 times the recommended dosage) to 400 mg. Mavorixafor steady state is reached after approximately 9 to 12 days at the highest approved recommended dosage in healthy subjects.
Absorption
Mavorixafor median (range) time to C max(T max) is 2.8 hours (1.9 to 4 hours) at the highest approved recommended dosage.
Effect of Food
High Fat Meal:Mavorixafor C maxdecreased by 66% and AUC decreased by 55% following single-dose administration of XOLREMDI 400 mg with a high‑fat meal (1000 calories, 50% fat) to healthy subjects.
Low Fat Meal:Mavorixafor C maxdecreased by 55% and AUC decreased by 51% following single-dose administration of XOLREMDI 400 mg with a low-fat meal (500 calories, 25% fat) to healthy subjects. In addition, a 14% higher mavorixafor C maxand 18% lower AUC was observed following single-dose administration of XOLREMDI 400 mg with a low-fat meal to healthy subjects after an overnight fast compared to fasting for an additional 4 hours after the XOLREMDI dose.
Distribution
Mavorixafor volume of distribution is 768 L. Mavorixafor is >93% bound to human plasma proteins in vitro.
Elimination
Mavorixafor's terminal half-life is 82 h (34%) with an apparent clearance of 62 L/h (40%) following single-dose administration of XOLREMDI 400 mg in health subjects. Mavorixafor exhibits at least partial nonlinear apparent clearance; however, this is not clinically significant at the approved recommended dosage.
Specific Populations
No clinically significant differences in the pharmacokinetics of mavorixafor were observed based on age (12 to 58 years), sex, or mild to moderate renal impairment (CLcr 30 to <90 mL/min as estimated by the Cockcroft-Gault formula). The effects of severe renal impairment (CLcr 15 to <30 mL/min), end-stage renal disease (CLcr <15 mL/min), and moderate to severe hepatic impairment on the pharmacokinetics of mavorixafor are unknown.
Lower body weight was associated with lower mavorixafor clearance. Mavorixafor exposures in patients with WHIM syndrome are comparable between those weighing 50 kg or less who receive 300 mg once daily and those weighing greater than 50 kg who receive 400 mg once daily. Following 400 mg once daily, median C maxand AUC is 22% and 30% lower, respectively, in patients with higher body weight (85 kg and above) compared to patients with average body weight (50 to less than 85 kg). The difference in exposure between patients with average body weight and patients with higher body weight is not expected to have a clinically significant impact on patient outcomes.
Drug Interaction Studies
Clinical Studies
Strong CYP3A4 Inhibitors:The systemic exposures of mavorixafor following concomitant administration of a single dose of XOLREMDI 200 mg (0.5 times the recommended dosage for adult and adolescent patients 12 years and older weighing over 50 kg) with 200 mg itraconazole (strong CYP3A4 inhibitor and P-gp inhibitor) dosed to steady state was similar to the mavorixafor systemic exposure from a single dose of XOLREMDI 400 mg administered alone in healthy subjects. These results suggest an approximate increase in mavorixafor exposure by 2-fold due to itraconazole.
CYP2D6 Substrates:Dextromethorphan (CYP2D6 substrate) C maxincreased by 6-fold (CI 90%: 5.1 to 8.3) and AUC by 9-fold (CI 90%: 6.5 to 12.3) following concomitant use with XOLREMDI 400 mg in healthy subjects.
CYP3A4 Substrates:Midazolam (CYP3A4 substrate) C maxincreased by 1.1-fold (CI 90%: 1.0 to 1.3) and AUC by 1.7-fold (CI 90%: 1.4 to 2.1) following concomitant use with XOLREMDI 400 mg in healthy subjects.
P-gp Substrates:
Digoxin:Digoxin C maxincreased by 1.5-fold (CI 90%: 1.3 to 1.8) and AUC increased by 1.6-fold (CI 90%: 1.4 to 1.9) following concomitant use of a single oral dose of a transporter cocktail containing 0.25 mg of digoxin with XOLREMDI dosed to steady state (400 mg/day) in healthy subjects.
In Vitro Studies
CYP450 Enzymes:Mavorixafor is a substrate of CYP3A4, CYP2D6, CYP3A5, and CYP2C19, but is not a substrate of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2E1, and CYP4A11. Mavorixafor is an inhibitor of CYP3A4 (time dependent), CYP3A5, CYP2D6, CYP2B6, CYP1A2, CYP2C8, CYP2C9, and CYP2C19. Mavorixafor is an inducer of CYP1A2, but not an inducer of CYP2B6, CYP2C8, CYP2C9 and CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with mavorixafor have not been conducted.
Mavorixafor was not genotoxic in an in vitrobacterial reverse mutation assay (Ames test), in an in vitrohuman lymphocyte culture chromosome aberration assay, or an in vivorat bone marrow micronucleus assay.
Fertility studies have not been conducted with mavorixafor. Significant tubular degeneration/atrophy was observed in the testes of dogs at clinical exposure.
-
14 CLINICAL STUDIES
The efficacy of XOLREMDI in patients aged 12 and older with WHIM syndrome was demonstrated in the 52-week, randomized, double-blind, placebo-controlled portion of Study 1 [NCT03995108]. Enrolled patients had a genotype-confirmed variant of CXCR4 consistent with WHIM syndrome, and a confirmed absolute neutrophil count (ANC) ≤400 cells/µL. Patients were permitted to continue (but not initiate) immunoglobulin therapy at the same dose. Use of other CXCR4 antagonists was not permitted. Baseline patient demographics are shown in Table 2.
Table 2: Baseline Demographic and Disease Characteristics in Patients with WHIM Syndrome (Study 1) Demographics and Disease Characteristics XOLREMDI
(N = 14)Placebo
(N = 17)Abbreviations: SD = standard deviation; Ig = immunoglobulin. Note: Percentages are calculated based on the number of patients within each characteristic as denominator. Demographics Age(years) Mean (SD) 22.1 (12.20) 30.9 (21.25) Age group, n (%) 12 to <18 years 7 (50) 8 (47.1) ≥18 years 7 (50) 9 (52.9) Sex, n (%) Male 5 (35.7) 8 (47.1) Female 9 (64.3) 9 (52.9) Race, n (%) White 13 (93) 16 (94) Asian 0 1 (6) Other 1 (7) 0 Ethnicity, n (%) Not Hispanic or Latino 13 (93) 17 (100) Hispanic or Latino 1 (7) 0 Disease Characteristics Baseline Ig use, n (%) Yes 6 (42.9) 8 (47.1) Baseline mean absolute neutrophil count (ANC)(cells/µL) Mean (SD) 155 (93.8) 281 (232.7) Baseline mean absolute lymphocyte count (ALC)(cells/µL) Mean (SD) 501 (204.8) 563 (199.1) Thirty-one patients were randomized 1:1 to receive either placebo (N=17) or XOLREMDI (N=14) once daily for 52 weeks. The efficacy of XOLREMDI in the treatment of patients with WHIM syndrome was based on improvement in absolute neutrophil counts (ANC), improvement in absolute lymphocyte counts (ALC), and a reduction in infections.
For ANC, the mean time (hours) above ANC threshold (TAT ANC) of 500 cells/µL over a 24-hour period was assessed 4 times throughout the study (every 3 months for 12 months). The results over the 52-week period showed that TAT ANCwas statistically significantly greater in patients treated with XOLREMDI (LS mean [SE] 15.0 [1.89] hours) compared with placebo (2.8 [1.52] hours) (p value <0.0001) (see Table 3and Figure 2).
Table 3 Mean Time (hours) Above ANC Threshold (TAT ANC) in Study 1 XOLREMDI
(N = 14)Placebo
(N = 17)Abbreviations: ANC = absolute neutrophil count; CI = confidence interval; LS = least squares; MMRM = mixed-model repeated measures; SD = standard deviation; SE = standard error; TAT = time above threshold of 500 cells/µL. - *
- The results are based on an MMRM analysis with time above threshold as a dependent variable; treatment, visit (Weeks 13, 26, 39 and 52), treatment*visit, Ig use (randomization strata), and baseline time above threshold as covariates; and patient as the repeated random effect.
TAT ANC(hours) Baseline Mean (SD) 0.0 (0.0) 3.6 (5.7) Overall MMRM results LS mean (SE) 15.0 (1.89) 2.8 (1.52) LS mean 95% CI (11.2, 18.9) (0.0, 5.9) Difference from placebo: LS mean difference (SE) 12.3 (2.49) - LS mean difference 95% CI (7.2, 17.4) - P-value * <0.0001 - Abbreviations: ANC = absolute neutrophil count; CI = confidence interval; LS = least squares; TAT = total time (hours) above threshold (500 cells/µL) in 24 hours.
1At Week 52, 3 of 17 placebo patients were given XOLREMDI in advance of their TAT measurements as they entered the open-label period of the study; one XOLREMDI patient did not take XOLREMDI. All data were included in the ITT analysis.Figure 2: TAT ANCOver Time (Hours) (LS Mean ± 95% CI) by Treatment Group (Study 1) 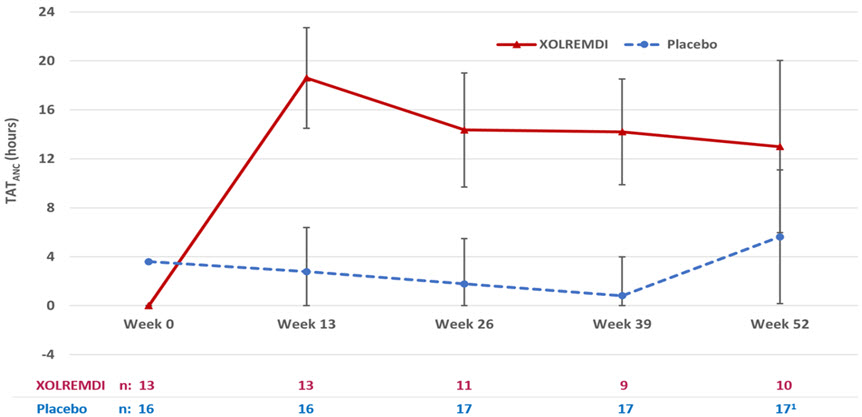
For ALC, the mean time (hours) above ALC threshold (TAT ALC) of 1,000 cells/µL over a 24-hour period was assessed 4 times throughout the study (every 3 months for 12 months). The results over the 52-week period showed that TAT ALCwas statistically significantly greater in patients treated with XOLREMDI (LS mean [SE] 15.8 [1.39] hours) compared with placebo (4.6 [1.15] hours) (p value <0.0001).
The efficacy of XOLREMDI was further assessed in a composite endpoint consisting of total infection score and total wart change score using a Win-Ratio method (Table 4). The Win-Ratio of 2.76 is the number of pairs of XOLREMDI-treated patient "wins" divided by the number of pairs of placebo patient "wins."
Table 4: Win-Ratio Analysis *for the Composite Clinical Efficacy Endpoint Based on Total Infection Score and Total Wart Change Score Category n † Win-Ratio
(95%CI)- *
- The method compared each XOLREMDI-treated patient to each placebo-treated patient in a pair-wise manner that proceeded in a hierarchical fashion using total infection score, followed by total wart change score if patients could not be differentiated based on total infection score. The total infection score was calculated by summing up the number of infection events weighted by severity and divided by the total exposure time (in years). Total wart change score was calculated by summing up the regional wart change scores from all 3 target regions (lesions).
- †
- n is number of wins.
XOLREMDI wins on total infection score 174 2.76 (1.60, 4.76) Placebo wins on total infection score 63 XOLREMDI wins on total wart change score 0 Placebo wins on total wart change score 0 None of the above (tie) 1 Analyses of the individual components of this composite endpoint showed an approximately 40% reduction of total infection score, weighted by infection severity, in XOLREMDI-treated patients compared with placebo-treated patients. The annualized infection rate was reduced approximately 60% in XOLREMDI-treated patients [LS mean (SE) 1.7(0.5)] compared with placebo-treated patients [LS mean (SE) 4.2(0.7)]. There was no difference in total wart change scores between the XOLREMDI and placebo treatment arms over the 52-week period.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XOLREMDI is supplied as an opaque white, hard gelatin capsule with a light blue cap, containing 100 mg of the active ingredient mavorixafor. The white capsule body is axially imprinted with "100 mg" in black ink, and the light blue capsule cap is axially imprinted with "MX4" in black ink.
XOLREMDI is supplied in child-resistant bottles as follows:
- 60 count– NDC 83296-100-60
- 120 count– NDC 83296-100-12
-
17 PATIENT COUNSELING INFORMATION
Administration
Advise patients to take XOLREMDI on an empty stomach after an overnight fast, 30 minutes before food. Advise patients to swallow the capsules whole and not to open, break, or chew capsules [see Dosage and Administration (2.1)and Clinical Pharmacology (12.3)] .
Embryo-Fetal Toxicity
XOLREMDI is expected to cause fetal harm. Advise females of reproductive potential to use an effective form of contraception during treatment with XOLREMDI and for three weeks after the final dose. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.1)and Use In Specific Populations (8.1)] .
Lactation
Advise females that breastfeeding is not recommended during treatment with XOLREMDI and for three weeks after the final dose [see Use in Specific Populations (8.2)] .
Drug Interactions
Advise patients to avoid taking dietary supplements that include goldenseal, as it is a strong CYP3A4 inhibitor and may increase risk of adverse reactions to XOLREMDI.
Advise patients to avoid taking dietary supplements that include St. John's Wort, as it is an inducer of CYP3A4 and may reduce the efficacy of XOLREMDI [see Drug Interactions (7.2)] .
Food Interactions
Advise patients to avoid eating or drinking products with grapefruit, as grapefruit is a strong CYP3A4 inhibitor and may increase the risk of adverse reactions to XOLREMDI [see Drug Interactions (7.1)] .
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
XOLREMDI
mavorixafor capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83296-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAVORIXAFOR (UNII: 0G9LGB5O2W) (MAVORIXAFOR - UNII:0G9LGB5O2W) MAVORIXAFOR 100 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color white (hard gelatin capsules with white body and light blue cap) Score no score Shape CAPSULE Size 1mm Flavor Imprint Code 100;mg;MX4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83296-100-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/21/2024 2 NDC:83296-100-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 05/21/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218709 05/21/2024 Labeler - X4 Pharmaceuticals, Inc. (080014773) Establishment Name Address ID/FEI Business Operations Catalent Greenville, Inc. 118812386 manufacture(83296-100) , analysis(83296-100) , pack(83296-100) , label(83296-100)


