Label: LAZCLUZE- lazertinib tablet, film coated
- NDC Code(s): 57894-080-60, 57894-080-90, 57894-240-30, 57894-240-99
- Packager: Janssen Biotech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LAZCLUZE safely and effectively. See full prescribing information for LAZCLUZE. LAZCLUZE™ (lazertinib) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELAZCLUZE, in combination with amivantamab, is indicated for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients for the first-line treatment of NSCLC with LAZCLUZE, in combination with amivantamab, based on the presence of EGFR exon 19 deletions or exon 21 L858R ...
-
3 DOSAGE FORMS AND STRENGTHSTablets - 80 mg tablets: yellow, oval film-coated tablet, debossed with "LZ" on one side and "80" on the other side. Each tablet contains 80 mg of lazertinib. 240 mg tablets: reddish purple ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Venous Thromboembolic Events - LAZCLUZE in combination with amivantamab can cause serious and fatal venous thromboembolic events (VTE), including deep venous thrombosis (DVT) and pulmonary ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: Venous Thromboembolic Events - [see - Warnings and Precautions (5.1)] Interstitial Lung ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on LAZCLUZE - CYP3A4 Inducers - Avoid concomitant use of LAZCLUZE with strong and moderate CYP3A4 inducers. Consider an alternate concomitant medication with no ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action - [see - Clinical Pharmacology (12.1)] , LAZCLUZE can cause fetal harm when ...
-
11 DESCRIPTIONLAZCLUZE™ tablets contain lazertinib, a kinase inhibitor for oral use. Lazertinib is present as lazertinib mesylate hydrate with a molecular weight of 668.77 and molecular formula of C - 30H ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lazertinib is a kinase inhibitor of epidermal growth factor receptor (EGFR) that inhibits EGFR exon 19 deletions and exon 21 L858R substitution mutations at lower ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with lazertinib. Lazertinib was not genotoxic in an in vitro bacterial reverse mutation ...
-
14 CLINICAL STUDIESThe efficacy of LAZCLUZE, in combination with amivantamab, was evaluated in MARIPOSA [NCT04487080], a randomized, active-controlled, multicenter trial. Eligible patients were required to have ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLAZCLUZE™ (lazertinib) tablets are available in the strengths and packages listed below: Tablet StrengthDescriptionPackage ConfigurationNDC Number - 80 mgYellow, oval, film-coated ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Venous Thromboembolic Events - Advise patients of the risks of serious and life threatening venous ...
-
SPL UNCLASSIFIED SECTIONProduct of Belgium - Manufactured for: Janssen Biotech, Inc. Horsham, PA 19044, USA - For patent information: www.janssenpatents.com - © Johnson & Johnson and its affiliates 2024
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - LAZCLUZE™ (laz-kluez) (lazertinib) tablets, for oral use - This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 240 mg Tablet Bottle CartonNDC 57894-240-30 - Lazcluze™ (lazertinib) tablets - 240 mg - Each film-coated tablet contains - 240 mg of lazertinib equivalent - to 281.58 mg lazertinib mesylate - (on ...
-
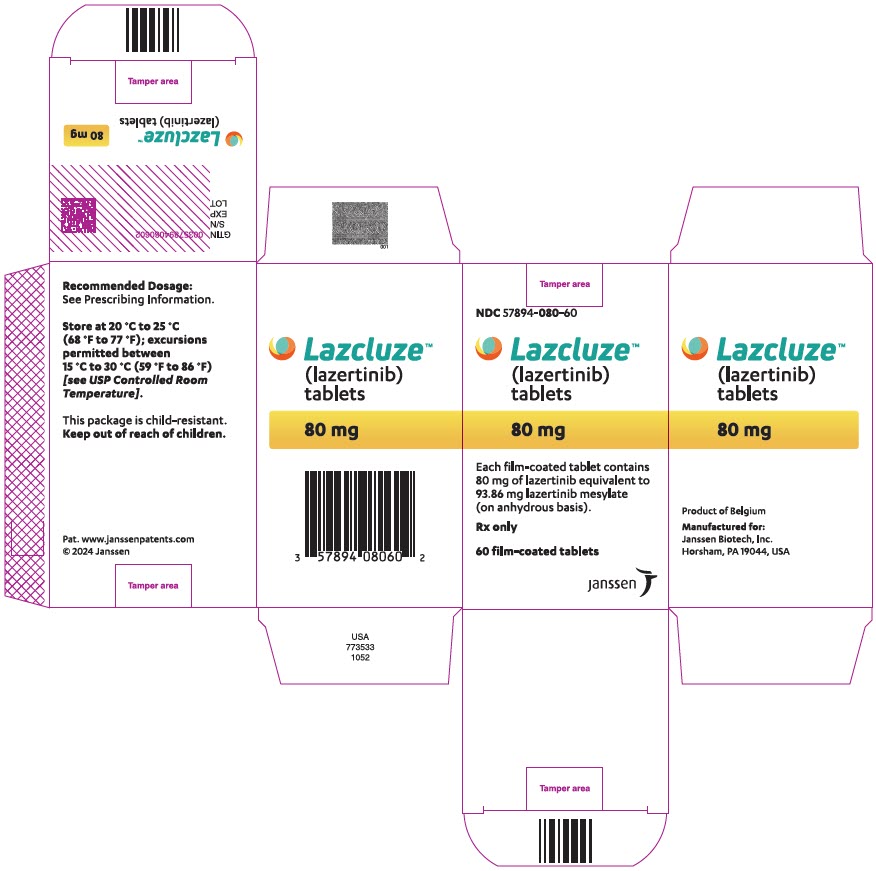
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle CartonNDC 57894-080-60 - Lazcluze™ (lazertinib) tablets - 80 mg - Each film-coated tablet contains - 80 mg of lazertinib equivalent to - 93.86 mg lazertinib mesylate - (on anhydrous ...
-
INGREDIENTS AND APPEARANCEProduct Information