Label: LUMISIGHT- pegulicianine injection, powder, lyophilized, for solution
- NDC Code(s): 82292-040-01, 82292-040-10
- Packager: Lumicell, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LUMISIGHT safely and effectively. See full prescribing information for LUMISIGHT.
LUMISIGHT™ (pegulicianine) for injection, for intravenous use
Initial U.S. Approval: 2024WARNING: ANAPHYLAXIS AND OTHER SERIOUS HYPERSENSITIVITY REACTIONS
See full prescribing information for complete boxed warning.
Serious hypersensitivity reactions, including anaphylaxis, can occur during or following administration. Anaphylaxis has occurred in 4/726 (0.6%) of patients in clinical studies. Before LUMISIGHT administration, assess all patients for any history of hypersensitivity reaction to contrast media or products containing polyethylene glycol (PEG). Always have emergency resuscitation drugs, equipment, and trained personnel available. Monitor all patients for hypersensitivity reactions. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy. (5.1).
INDICATIONS AND USAGE
LUMISIGHT is an optical imaging agent indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
For injection: lyophilized powder in a single-dose vial delivering 39 mg of pegulicianine after reconstitution. (3)
CONTRAINDICATIONS
History of hypersensitivity reaction to pegulicianine. (4)
WARNINGS AND PRECAUTIONS
- Risk of Misdiagnosis: Absence of signal in the surgical field does not rule out the presence of cancer. Positive signal may be seen in non-cancerous tissue. (5.2)
- Interference from Dyes Used for Sentinel Lymph Node Mapping: Avoid administration of dyes before imaging the lumpectomy cavity in patients receiving LUMISIGHT. (5.3, 7)
ADVERSE REACTIONS
Most common adverse reactions (≥1%) were hypersensitivity and chromaturia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lumicell at 1-833-458-6387 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ANAPHYLAXIS AND OTHER SERIOUS HYPERSENSITIVITY REACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Preparation of LUMISIGHT
2.3 Administration
2.4 Imaging
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Other Serious Hypersensitivity Reactions
5.2 Risk of Misdiagnosis
5.3 Interference from Dyes Used for Sentinel Lymph Node Mapping
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ANAPHYLAXIS AND OTHER SERIOUS HYPERSENSITIVITY REACTIONS
Serious hypersensitivity reactions, including anaphylaxis, can occur during or following administration of LUMISIGHT. Anaphylaxis occurred in 4/726 (0.6%) of patients in clinical studies. Signs and symptoms associated with other hypersensitivity reactions included pruritus, urticaria, hypotension, lip swelling, erythema, anxiety, chest pain, cyanosis, dizziness, dyspnea, headache, hypoesthesia, hyperventilation, maculopapular rash, nausea, paresthesia, visual changes, and vomiting.
- Before LUMISIGHT administration, assess all patients for any history of hypersensitivity reaction to contrast media or products containing polyethylene glycol (PEG).
- Always have emergency resuscitation drugs, equipment, and trained personnel promptly available.
- Monitor all patients for hypersensitivity reactions. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy.
- LUMISIGHT is contraindicated in patients with a history of hypersensitivity reactions to pegulicianine [see Warning and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of LUMISIGHT is 1 mg/kg actual body weight by intravenous injection over 3 minutes administered 2 hours to 6 hours prior to imaging.
2.2 Preparation of LUMISIGHT
Important Preparation Information
- Prior to reconstitution, store vials in the freezer at -25°C to -15°C (-13°F to 5°F). Protect from light.
- Use aseptic technique for the preparation of LUMISIGHT.
- The recommended dose depends on the individual patient’s weight. Multiple vials of LUMISIGHT may need to be reconstituted to achieve the individual patient dose.
- Only use 0.45% Sodium Chloride Injection, USP for reconstitution of LUMISIGHT to prevent high osmolality.
Preparation Instructions
- Calculate the dose (1 mg/kg) and the total volume (mL) of LUMISIGHT based on the individual patient’s weight.
- Obtain the number of vials required to administer the patient dose.
- Allow the vials to acclimate to room temperature between 20°C to 25°C (68°F to 77°F) for approximately 5 minutes.
- Reconstitute each vial of LUMISIGHT with 4 mL of 0.45% Sodium Chloride Injection, USP to permit withdrawal of 3.9 mL of LUMISIGHT 10 mg/mL.
- Visually inspect the reconstituted solution. It should be a clear, dark blue-colored solution free of particulate matter. Discard if there is any discoloration or particulate matter.
- If not immediately used, store the reconstituted LUMISIGHT vial at room temperature at 20°C to 25°C (68°F to 77°F) and use within 4 hours, or store the reconstituted LUMISIGHT vial in the refrigerator at 2°C to 8°C (35°F to 46°F) and use within 24 hours. Protect from light.
- Each vial of LUMISIGHT is for a single dose. Discard unused portion.
2.3 Administration
- Withdraw the calculated volume from the appropriate number of vials into one syringe for administration of one single dose of 1 mg/kg. Verify that the syringe contains the intended volume.
- Prior to administration of LUMISIGHT, flush the peripheral intravenous (IV) line with 10 mL to 20 mL of 0.9% Sodium Chloride Injection, USP.
- Administer LUMISIGHT as an IV injection over 3 minutes beginning 2 hours to 6 hours prior to imaging with the Lumicell DVS.
- After administration of LUMISIGHT, flush the IV line with 10 mL to 20 mL of 0.9% Sodium Chloride Injection, USP.
2.4 Imaging
LUMISIGHT is used with the Lumicell Direct Visualization System (DVS) or other fluorescence imaging device that is FDA-approved for specific use with pegulicianine in the indicated population. The device provides illumination to excite the fluorescent components of pegulicianine and collects images showing pegulicianine’s fluorescence emission. Regions suspected to contain cancerous tissue are highlighted as positive signals on the Lumicell DVS display [see Clinical Studies (14)].
Training on the use of the device is essential prior to employing the system in a lumpectomy procedure. Please refer to the device labeling for details on how to use the device and for training information.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
LUMISIGHT is contraindicated in patients with a history of hypersensitivity reaction to pegulicianine. Reactions have included anaphylaxis [see Warning and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Other Serious Hypersensitivity Reactions
Prepare for the possibility of drug hypersensitivity reactions (including anaphylaxis), which can occur during or following administration, and take the necessary precautions.
In clinical studies, 4 of 726 (0.6%) patients treated with LUMISIGHT experienced signs and symptoms consistent with anaphylaxis. The onset was during administration in three patients. Signs and symptoms associated with other hypersensitivity reactions included pruritus, urticaria, hypotension, lip swelling, erythema, anxiety, chest pain, cyanosis, dizziness, dyspnea, headache, hypoesthesia, hyperventilation, maculopapular rash, nausea, paresthesia, visual changes, and vomiting [see Adverse Reactions (6.1)].
Before LUMISIGHT administration, assess all patients for any history of hypersensitivity reaction to contrast media or products containing polyethylene glycol (PEG), as these patients may have an increased risk for hypersensitivity reaction to LUMISIGHT. In clinical studies, three out of four patients that experienced anaphylaxis did not have a history of hypersensitivity reaction to contrast media or products containing PEG.
Always have emergency resuscitation drugs, equipment, and trained personnel available. Monitor all patients for hypersensitivity reactions through symptom reporting, direct observation, and vital sign measurements. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy. LUMISIGHT is contraindicated in patients with a history of hypersensitivity reaction to pegulicianine [see Contraindications (4)].
5.2 Risk of Misdiagnosis
False positive and false negative findings may occur during use of LUMISIGHT to detect residual cancer. Absence of signal in the lumpectomy cavity does not rule out the presence of residual cancer. Additionally, positive signal has been observed in some non-cancerous tissue [see Clinical Studies (14)].
5.3 Interference from Dyes Used for Sentinel Lymph Node Mapping
Blue dyes used for sentinel lymph node (SLN) mapping procedures interfere with LUMISIGHT imaging. The potential of other dyes to interfere with LUMISIGHT imaging has not been evaluated. Avoid administration of dyes used for SLN mapping procedures before imaging the lumpectomy cavity in patients receiving LUMISIGHT [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
The following clinically important adverse reactions are described elsewhere in the labeling:
- Anaphylaxis and Other Serious Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LUMISIGHT was evaluated in 726 patients who received a single dose of 1 mg/kg of LUMISIGHT. Among these 726 patients, 703 (97%) had breast cancer and 23 (3%) had other types of cancer. The mean age of the patients was 62 years (range: 36 years to 95 years), and 98% of them were female. Distribution by race was 82% White, 7% Black or African American, 6% Asian, and 5% other or unreported. Distribution by ethnicity was 3% Hispanic/Latino, 93% non-Hispanic/Latino, and 4% unknown or unreported.
Adverse reactions occurring in ≥ 1% of patients receiving LUMISIGHT were hypersensitivity (1.4%, including anaphylaxis [4 out of 726]) and chromaturia (85%). Chromaturia resolved within 48 hours after administration in 93% of patients, with the longest time to resolution of 15 days.
Adverse reactions occurring in use < 1% of patients were skin discoloration after extravasation, nausea, dyspnea, pyrexia, and vomiting.
-
7 DRUG INTERACTIONS
Blue dyes used for SLN mapping procedures generate a fluorescent signal that interferes with the signal from LUMISIGHT when injected into the breast prior to imaging with LUMISIGHT. The potential of other dyes to interfere with LUMISIGHT imaging has not been evaluated. Avoid administration of dyes used for SLN mapping procedure before imaging the lumpectomy cavity in patients receiving LUMISIGHT.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on pegulicianine use in pregnant women to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Animal reproduction studies were not conducted with pegulicianine.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of pegulicianine or its metabolites in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LUMISIGHT and any potential adverse effects on the breastfed infant from LUMISIGHT or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of LUMISIGHT in pediatric patients have not been established.
8.5 Geriatric Use
Of 703 patients in clinical studies of LUMISIGHT for breast cancer who received the recommended dose of 1 mg/kg, 44% were 65 years of age and over, while 8% were 75 and over. No overall differences in safety or effectiveness have been observed between these patients and younger adult patients.
-
11 DESCRIPTION
LUMISIGHT (pegulicianine for injection) is an optical imaging agent for intravenous use.
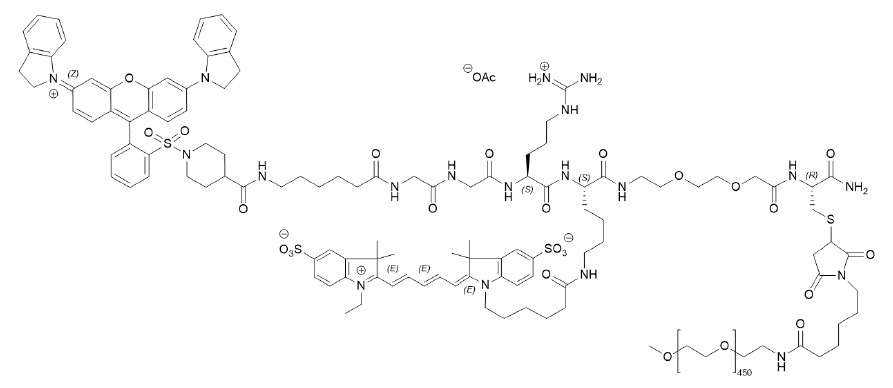
The chemical name of pegulicianine acetate is N-[6-(1-{2-[3,6-bis(2,3-dihydro-1H-indol-1-yl)xanthylium-9-yl]benzene-1-sulfonyl}piperidine-4-carboxamido)hexanoyl]glycylglycyl-L-arginyl-N6-(6-{2-[(1E,3E,5Z)-5-(1-ethyl-3,3-dimethyl-5-sulfonato-1,3-dihydro-2H-indol-2-ylidene)penta-1,3-dien-1-yl]-3,3-dimethyl-5-sulfonato-3H-indol-1-ium-1-yl}hexanoyl)-Llysyl-[2-(2-aminoethoxy)ethoxy]acetyl-S-[(3RS)-1-{6-[α-methylpoly(oxyethylene)-ω-amino]-6-oxohexyl}-2,5-dioxopyrrolidin-3-yl]-L-cysteinamide acetate with a molecular formula of C116H147N19O23S4(C2H4O)n, with n of approximately 450, a molecular weight of 20-25 kDa, and the following structural formula:
Figure 1: Molecular structure of pegulicianine acetate
LUMISIGHT is supplied as a sterile, dark blue lyophilized powder. Each vial contains 40 mg of pegulicianine (equivalent to 40.1 mg of pegulicianine acetate) and the following inactive ingredients: 3.2 mg of dibasic sodium phosphate heptahydrate, 39.5 mg of mannitol, and 3.8 mg of monobasic sodium phosphate monohydrate to permit withdrawal of 3.9 mL of pegulicianine 10 mg/mL upon reconstitution with 4 mL of 0.45% sodium chloride injection, USP. The pH of the reconstituted solution is 6 to 7.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pegulicianine is a prodrug that is optically inactive when intact and produces a fluorescent signal after its peptide chain is cleaved by cathepsins and matrix metalloproteases (MMPs). The levels of these enzymes are higher in and around tumor and tumor-associated cells than normal cells.
This enzymatic cleavage results in “fragment 2” and “fragment 3”, which are optically active metabolites that emit fluorescence, as well as “fragment 1” containing the fluorescence quencher that keeps the intact molecule optically inactive. “Fragment 2” and “fragment 3” absorb light in the visible light region with a peak absorption at 650 nm and fluoresce with a peak emission at 675 nm.
12.2 Pharmacodynamics
The relationships between the plasma concentrations of the optically active metabolites and the time course of pharmacodynamic response have not been fully characterized.
12.3 Pharmacokinetics
Elimination
Metabolism
Pegulicianine is cleaved by cathepsins and matrix metalloproteases to the active metabolites “fragment 2” and “fragment 3.” Pegulicianine undergoes minimal hepatic metabolism in vitro.
Excretion
Pegulicianine excretion pathway in humans is unknown. Observed blue/green discoloration of urine (chromaturia) in subjects receiving pegulicianine suggests that pegulicianine and/or its metabolites may be excreted renally.
Specific Populations
Patients with Renal Impairment
Mild renal impairment (RI) does not appear to have a clinically meaningful effect on the pharmacokinetics, safety, and efficacy of pegulicianine and fragment 3; fragment 2 data are not available. The effect of moderate or severe RI on the pharmacokinetics, safety, and efficacy of pegulicianine, fragment 2, and fragment 3 has not been evaluated.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies in animals have been conducted to evaluate the carcinogenic potential of pegulicianine.
The nonfluorescent major metabolite of pegulicianine was not mutagenic in nonclinical tests, i.e., in vitro reverse mutation test in bacteria (Ames test), in vitro chromosomal aberration test in human peripheral blood lymphocytes, and in vivo (mice) micronucleus test after intravenous doses up to 11.24 mg/kg (225 times the pegulicianine clinical dose of 1 mg/kg).
No reproductive and developmental toxicity studies in animals have been performed to evaluate the effects of pegulicianine on fertility.
-
14 CLINICAL STUDIES
The efficacy and safety of LUMISIGHT for the intraoperative detection of cancerous tissue within the resection cavity following removal of the primary specimen during lumpectomy surgery in patients with breast cancer were evaluated in a randomized, multicenter, intra-patient controlled clinical trial NCT03686215. A total of 406 adult patients with confirmed invasive breast cancer, ductal carcinoma in situ (DCIS), or both received 1 mg/kg LUMISIGHT by intravenous injection 2 hours to 6 hours prior to imaging with the Lumicell DVS. Among them, 357 patients underwent LUMISIGHT-guided imaging after completion of the standard of care lumpectomy procedure. Patients who received neoadjuvant chemotherapy or radiotherapy prior to surgery were excluded from study. After the standard of care (SOC) lumpectomy was completed, the lumpectomy cavity was divided into six regions based on anatomic orientation and each region was imaged with the Lumicell DVS. When positive fluorescence signal was detected, the tissue was resected with a cavity shave procedure and the region was re-imaged. A maximum of two LUMISIGHT-guided shaves were obtained from a single region. Histopathology analysis of excised tissue served as the reference standard. Cavity regions that did not have a LUMISIGHT-guided cavity shave or tissue from a second surgery for a reference standard were assigned the margin status of the corresponding outermost surface of the lumpectomy specimen or SOC cavity shave, with a positive margin defined as tumor on ink for invasive cancers (with or without DCIS) or within 2 mm of the inked margin for DCIS alone.
The mean age of patients was 62 years (range: 36 years to 83 years). Distribution by race was 82% White, 7% Black or African American, 6% Asian, and 5% other. Distribution by ethnicity was 3% Hispanic/Latino, 94% non-Hispanic/Latino, and 3% unknown or unreported. In the study group, 70% of patients had invasive ductal carcinoma (with or without DCIS), 20% had DCIS only, 10% had invasive lobular carcinoma (with or without DCIS), and <1% had both invasive ductal and invasive lobular carcinoma.
The study assessed the proportion of patients receiving LUMISIGHT who had residual cancer detected and removed using the Lumicell DVS after completion of SOC lumpectomy. One hundred and sixty-six (166) of 357 (46%) patients had at least one LUMISIGHT-guided shave. A total of 27 of 357 patients had residual cancer confirmed by histopathology in at least one LUMISIGHT-guided shave (7.6%; 95% CI: 5.0%, 10.8%).
Table 1 shows sensitivity and specificity of LUMISIGHT for residual breast cancer after completion of SOC lumpectomy calculated from the 2,346 evaluable images in the study. Regions of the lumpectomy cavity from which LUMISIGHT-guided shaves were taken contributed more than one image to the analysis.
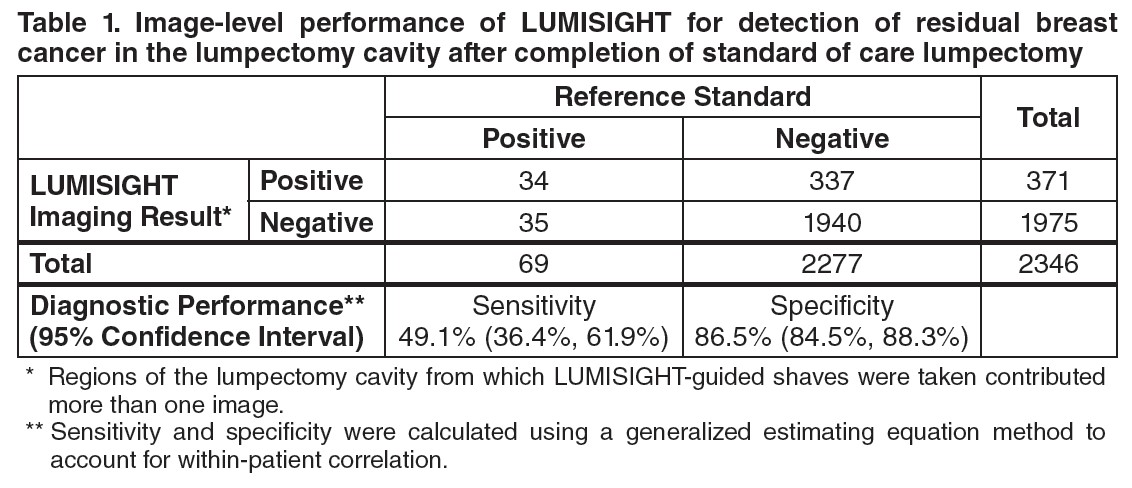
A total of 155 of 357 (43%) patients had at least one false positive image, and 28 of 357 (8%) patients had at least one false negative image. Among the 166 patients with at least one LUMISIGHT-guided shave, the mean total LUMISIGHT-guided shave volume was 22 cm3 ± 20 cm3, accounting for 20% ± 15% of the total volume of resection.
After completion of SOC lumpectomy, prior to LUMISIGHT-guided tissue removal, 62 of 357 patients (17%) had at least one cancer-positive margin. After LUMISIGHT-guided surgery, 9 of these 62 (15%) patients changed to having all cancer-negative margins, and 2 of the remaining 295 (1%) patients changed from having all cancer-negative margins to having at least one cancer-positive margin.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How supplied
LUMISIGHT (pegulicianine) for injection is supplied as a dark blue lyophilized powder for reconstitution in a clear, glass single-dose vial in cartons of 10 vials (NDC 82292-040-10). After reconstitution, each vial delivers 39 mg pegulicianine.
Storage and Handling
Store vials of LUMISIGHT frozen at -25°C to -15°C (-13°F to 5°F) in the original carton to protect from light.
-
17 PATIENT COUNSELING INFORMATION
Anaphylaxis and Other Serious Hypersensitivity Reactions
Inform patients of the risk of hypersensitivity reactions, including anaphylaxis, and instruct them to alert healthcare providers immediately if they experience signs and symptoms of a hypersensitivity reaction [see Warnings and Precautions (5.1)].
Urine Discoloration
Inform patients that LUMISIGHT may cause blue discoloration of the urine that in most cases will resolve within 48 hours [see Adverse Reactions (6.1)].
Skin Discoloration After Extravasation
Inform patients that if extravasation occurs, it may result in blue discoloration of the skin at the injection site that will be evident for several weeks [see Adverse Reactions (6.1)].
- SPL UNCLASSIFIED SECTION
-
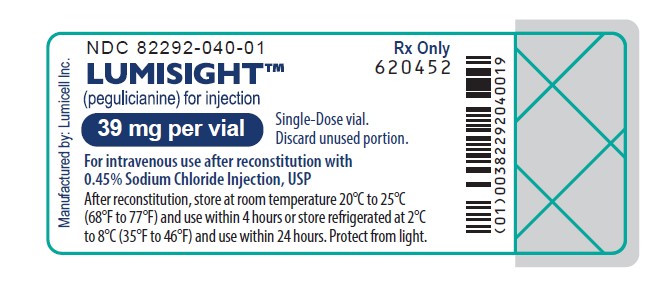
PRINCIPAL DISPLAY PANEL - Vial Label
Rx Only
NDC 82292-040-01LUMISIGHT
(pegulicianine for injection)
39 mg per vialSingle-Dose vial.
Discard unused portion.For intravenous use after reconstitution with
0.45% Sodium Chloride Injection, USPAfter reconstitution, store at room temperature 20°C to 25°C
(68°F to 77°F) and use within 4 hours or store refrigerated at 2°C
to 8°C (35°F to 46°F) and use within 24 hours. Protect from light.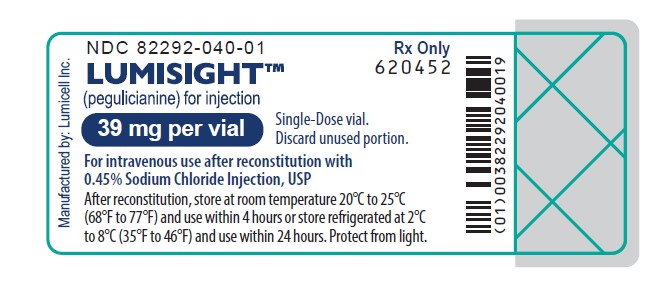
-
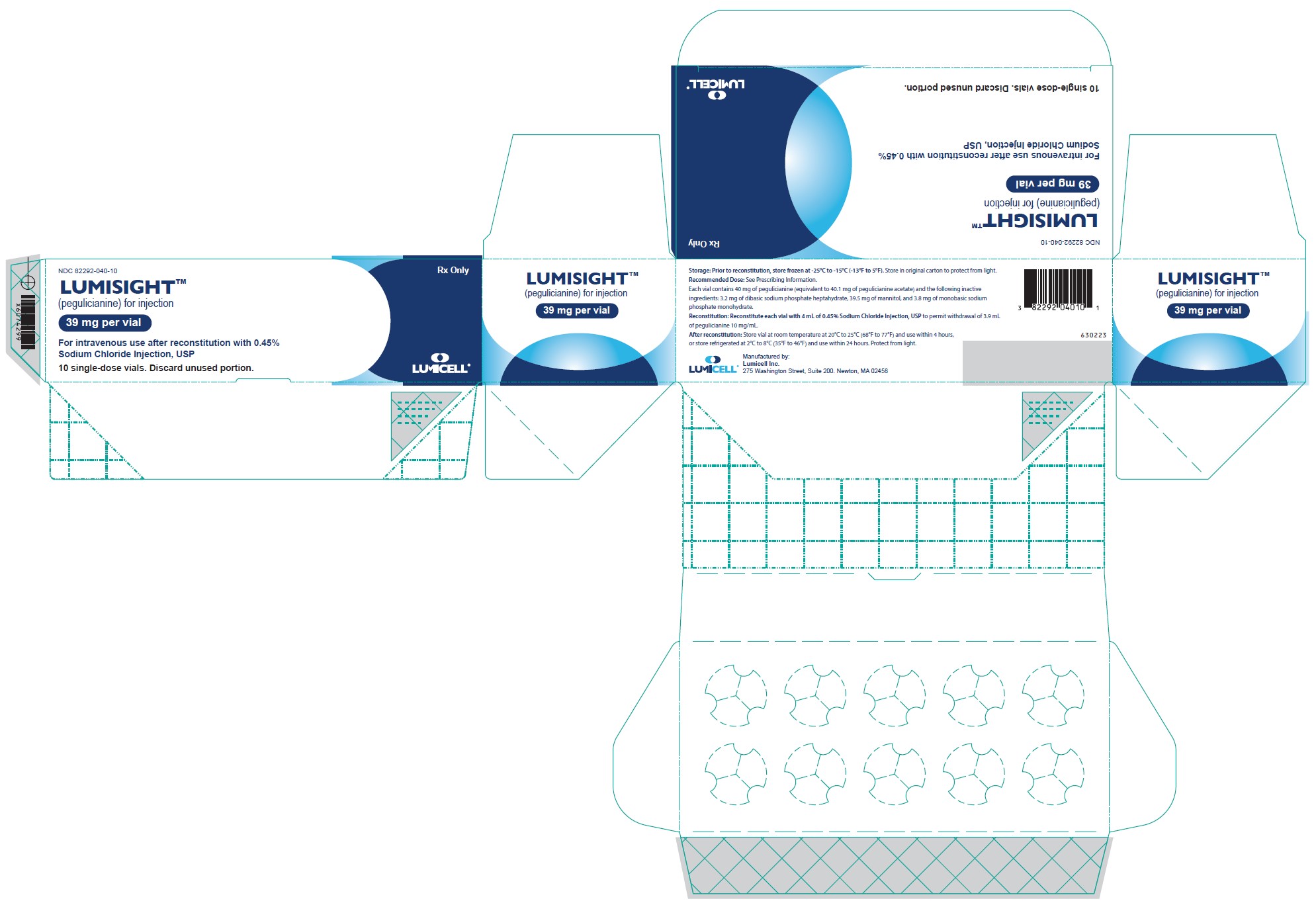
PRINCIPAL DISPLAY PANEL - Carton Label
Rx Only
NDC 82292-040-10LUMISIGHT
(pegulicianine for injection)
39 mg per vialSingle-Dose vial.
Discard unused portion.For intravenous use after reconstitution with
0.45% Sodium Chloride Injection, USP10 single-dose vials. Discard unused portion.
Storage: Prior to reconstitution, store frozen at -25°C to -15°C (-13°F to 5°F). Store in original carton to protect from light.
Recommended Dose: See Prescribing Information.
Each vial contains 40 mg of pegulicianine (equivalent to 40.1 mg of pegulicianine acetate) and the following inactive
ingredients: 3.2 mg of dibasic sodium phosphate heptahydrate, 39.5 mg of mannitol, and 3.8 mg of monobasic sodium
phosphate monohydrate.Reconstitution: Reconstitute each vial with 4 mL of 0.45% Sodium Chloride Injection, USP to permit withdrawal of 3.9 mL
of pegulicianine 10 mg/mL.After reconstitution: Store vial at room temperature at 20°C to 25°C (68°F to 77°F) and use within 4 hours,
or store refrigerated at 2°C to 8°C (35°F to 46°F) and use within 24 hours. Protect from light.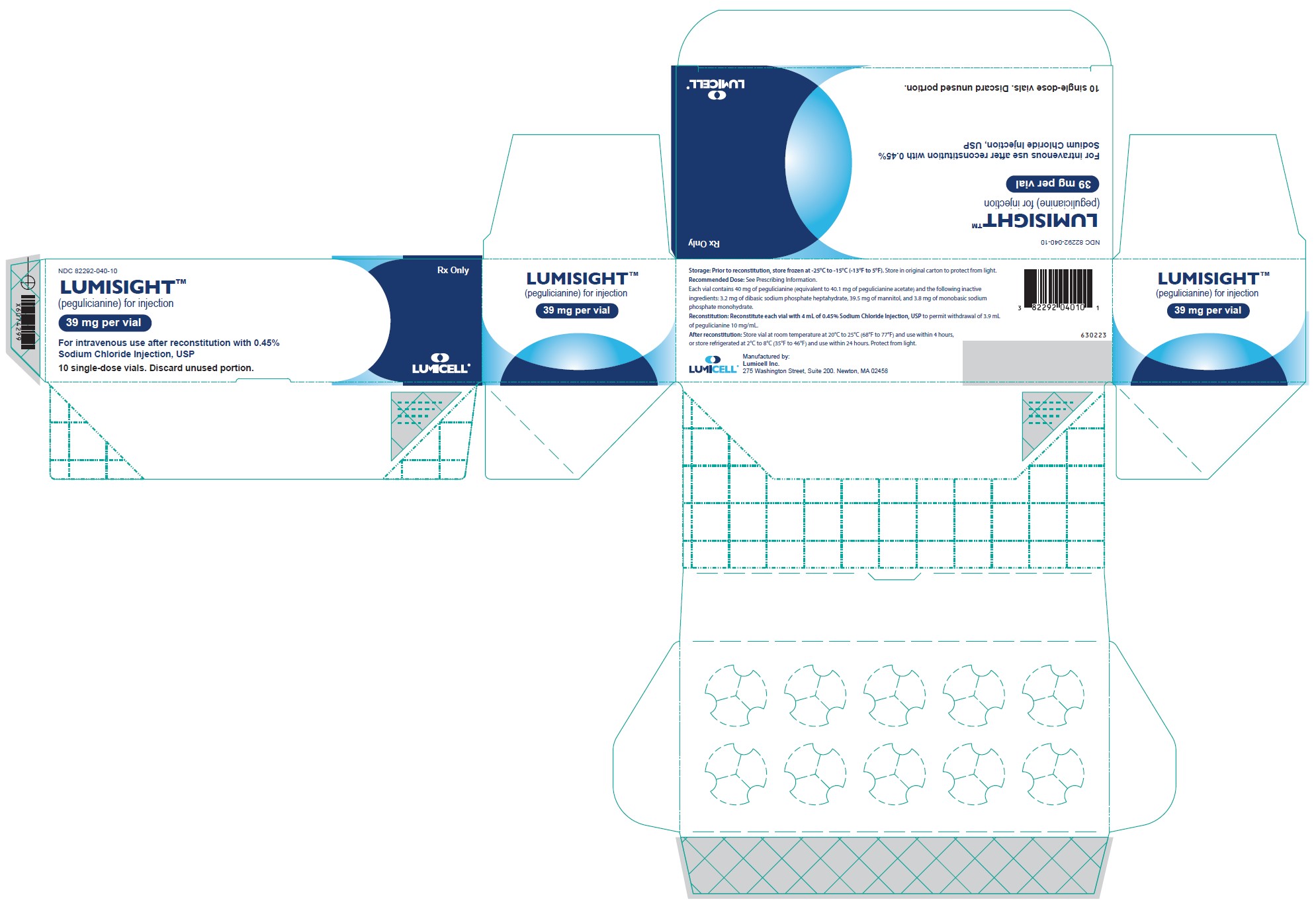
-
INGREDIENTS AND APPEARANCE
LUMISIGHT
pegulicianine injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82292-040 Route of Administration Intravenous Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEGULICIANINE (UNII: T6HE85WN0Q) (Pegulicianine - UNII:T6HE85WN0Q) Pegulicianine 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82292-040-10 10 in 1 CARTON 04/17/2024 1 NDC:82292-040-01 3.9 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214511 04/17/2024 Labeler - Lumicell, Inc. (832329788)