Label: BESPONSA- inotuzumab ozogamicin injection, powder, lyophilized, for solution
- NDC Code(s): 0008-0100-01
- Packager: Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BESPONSA™ safely and effectively. See full prescribing information for BESPONSA. BESPONSA (inotuzumab ozogamicin) for injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)HEPATOTOXICITY, INCLUDING VOD - • Hepatotoxicity, including fatal and life-threatening VOD occurred in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) who received ...
WARNING: HEPATOTOXICITY, INCLUDING HEPATIC VENO-OCCLUSIVE DISEASE (VOD) (ALSO KNOWN AS SINUSOIDAL OBSTRUCTION SYNDROME) and INCREASED RISK OF POST-HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT) NON-RELAPSE MORTALITY
HEPATOTOXICITY, INCLUDING VOD
- •
- Hepatotoxicity, including fatal and life-threatening VOD occurred in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) who received BESPONSA. The risk of VOD was greater in patients who underwent HSCT after BESPONSA treatment; use of HSCT conditioning regimens containing 2 alkylating agents and last total bilirubin level ≥ upper limit of normal (ULN) before HSCT were significantly associated with an increased risk of VOD.
- •
- Other risk factors for VOD in patients treated with BESPONSA included ongoing or prior liver disease, prior HSCT, increased age, later salvage lines, and a greater number of BESPONSA treatment cycles.
- •
- Elevation of liver tests may require dosing interruption, dose reduction, or permanent discontinuation of BESPONSA. Permanently discontinue treatment if VOD occurs. If severe VOD occurs, treat according to standard medical practice [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
CloseINCREASED RISK OF POST-HSCT NON-RELAPSE MORTALITY
- •
- There was higher post-HSCT non-relapse mortality rate in patients receiving BESPONSA, resulting in a higher Day 100 post-HSCT mortality rate [see Warnings and Precautions (5.2)].
-
1. INDICATIONS AND USAGEBESPONSA is indicated for the treatment of relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients 1 year and older.
-
2. DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - • Pre-medicate before each dose [see Dosage and Administration (2.2)]. • Administer by intravenous infusion only. • For the first cycle, the recommended total dose of ...
-
3. DOSAGE FORMS AND STRENGTHSFor injection: 0.9 mg as a white to off-white lyophilized powder in a single-dose vial for reconstitution and further dilution.
-
4. CONTRAINDICATIONSNone.
-
5. WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity, Including Hepatic Veno-occlusive Disease (VOD) (also known as Sinusoidal Obstruction Syndrome) BESPONSA can cause hepatotoxicity, including VOD. In adult patients in the ...
-
6. ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: • Hepatotoxicity, including hepatic VOD (also known as SOS) [see Warnings and Precautions ...
-
7. DRUG INTERACTIONSDrugs That Prolong the QT Interval - Concomitant use of BESPONSA with drugs known to prolong the QT interval or induce Torsades de Pointes may increase the risk of a clinically significant QTc ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings from animal studies [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)], BESPONSA can cause ...
-
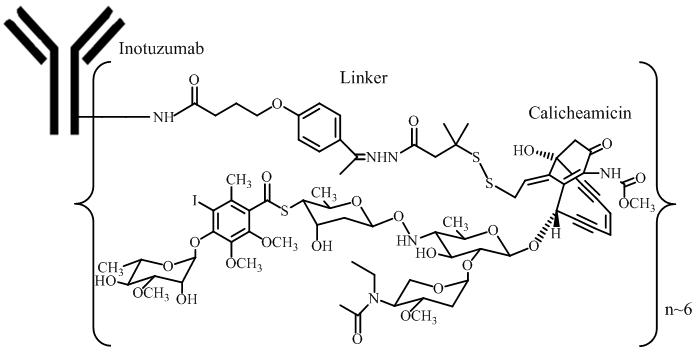
11. DESCRIPTIONInotuzumab ozogamicin is a CD22-directed antibody and cytotoxic-drug conjugate (ADC) consisting of 3 components: 1) the recombinant humanized immunoglobulin class G subtype 4 (IgG4) kappa antibody ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Inotuzumab ozogamicin is a CD22-directed antibody drug conjugate (ADC). Inotuzumab recognizes human CD22. The small molecule, N-acetyl-gamma-calicheamicin, is a ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Formal carcinogenicity studies have not been conducted with inotuzumab ozogamicin. In toxicity studies, rats were dosed weekly for 4 or ...
-
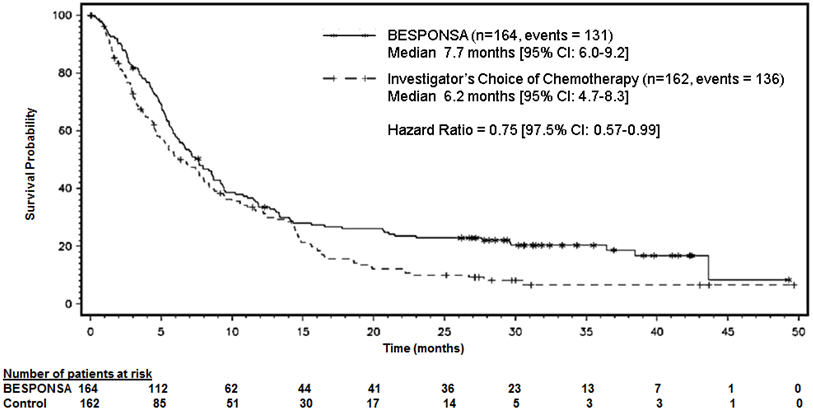
14. CLINICAL STUDIESRelapsed or Refractory ALL - INO-VATE ALL Study – Adult Patients - The safety and efficacy of BESPONSA were evaluated in INO-VATE ALL (NCT01564784) a randomized (1:1), open‑label ...
-
15. REFERENCES1. OSHA Hazardous Drugs. OSHA. https://www.osha.gov/hazardous-drugs
-
16. HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - BESPONSA (inotuzumab ozogamicin) for injection is supplied as a white to off-white lyophilized powder in a single-dose vial for reconstitution and further dilution. Each vial ...
-
17. PATIENT COUNSELING INFORMATIONHepatotoxicity, Including Hepatic Veno-occlusive Disease (VOD) (also known as Sinusoidal Obstruction Syndrome) Inform patients that liver problems, including severe, life-threatening ...
-
SPL UNCLASSIFIED SECTIONThis product's label may have been updated. For current full prescribing information, please visit www.BESPONSA.com. US License No. 003 - LAB-0763-3.0
-
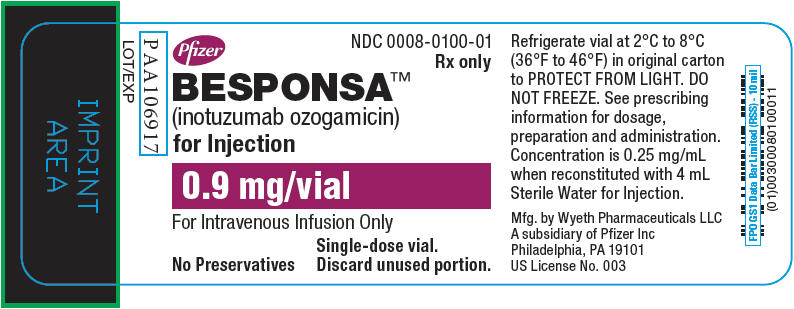
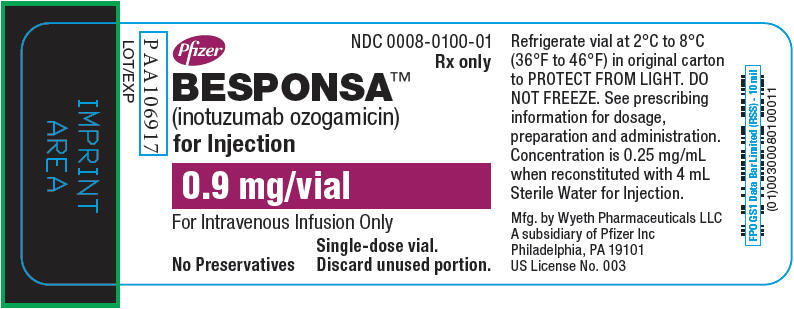
PRINCIPAL DISPLAY PANEL - 0.9 mg Vial LabelPfizer - NDC 0008-0100-01 - Rx only - BESPONSA™ (inotuzumab ozogamicin) for Injection - 0.9 mg/vial - For Intravenous Infusion Only - No Preservatives - Single-dose vial. Discard unused ...
-
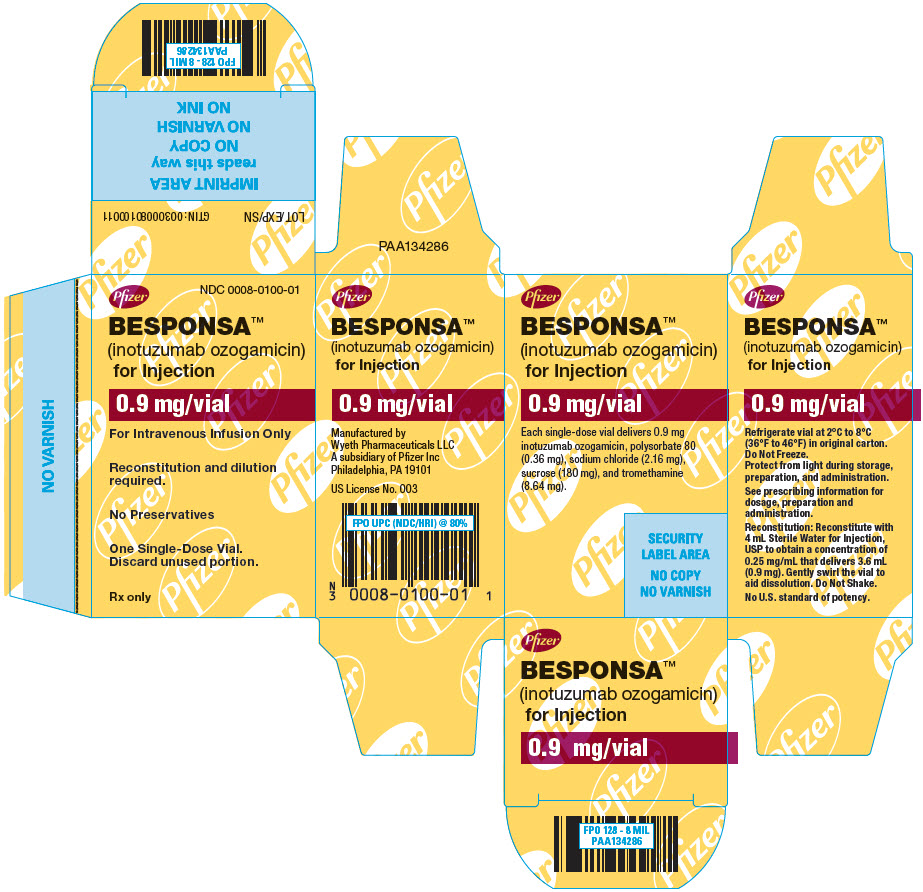
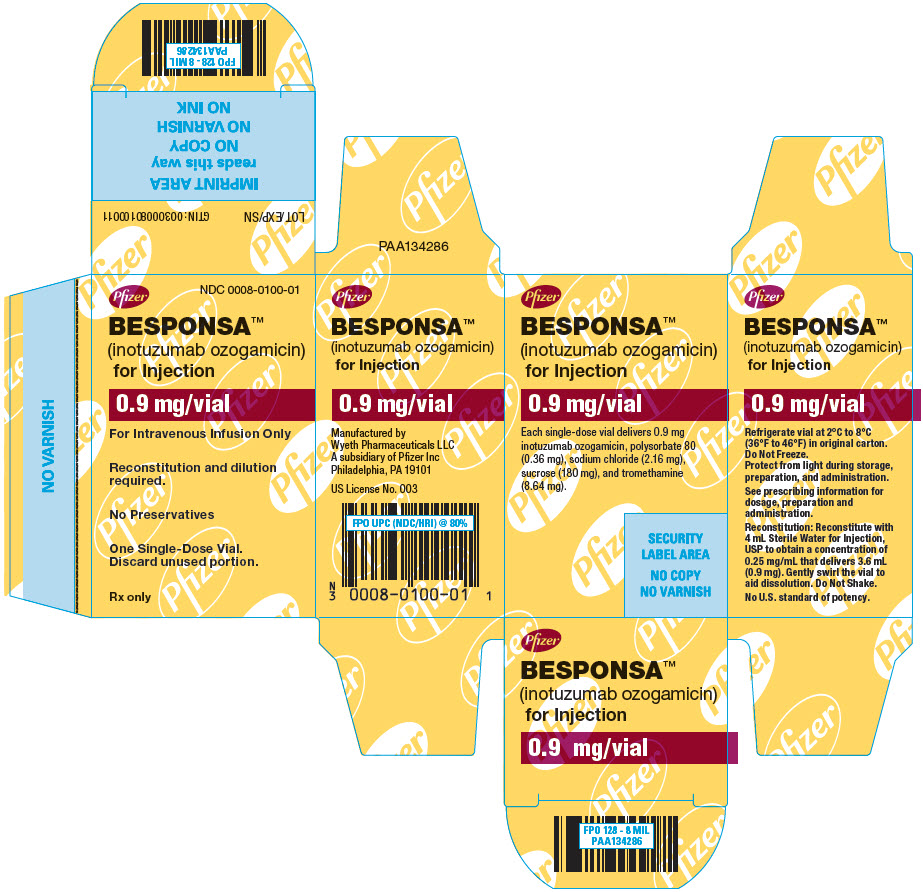
PRINCIPAL DISPLAY PANEL - 0.9 mg Vial CartonPfizer - NDC 0008-0100-01 - BESPONSA™ (inotuzumab ozogamicin) for Injection - 0.9 mg/vial - For Intravenous Infusion Only - Reconstitution and dilution - required. No Preservatives - One ...
-
INGREDIENTS AND APPEARANCEProduct Information