Label: ZENRELIA- ilunocitinib tablet
- NDC Code(s): 58198-5536-1, 58198-5536-2, 58198-5536-3, 58198-5537-1, view more
- Packager: Elanco US Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated September 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Zenrelia™ (ilunocitinib tablets) Immunomodulator - For oral use in dogs only - CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
BOXED WARNING
(What is this?)
WARNING: VACCINE-INDUCED DISEASE AND INADEQUATE IMMUNE RESPONSE TO VACCINES
Based on results of the vaccine response study, dogs receiving Zenrelia are at risk of fatal vaccine-induced disease from modified live virus vaccines and inadequate immune response to any vaccine. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination (see Warnings and Target Animal Safety).
Close -
DESCRIPTION
Zenrelia is a synthetic Janus kinase (JAK) inhibitor. Zenrelia is a member of the pyrimidines class of JAK inhibitors and its chemical name is 2-[1-cyclopropylsulfonyl-3-[4-(7H-pyrrolo[2,3-d ...
-
INDICATIONS Zenrelia is indicated for control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age.
-
DOSAGE AND ADMINISTRATION The dose of Zenrelia (ilunocitinib tablets) is 0.27 to 0.36 mg ilunocitinib/lb (0.6 to 0.8 mg ilunocitinib/kg) body weight, administered orally, once daily, with or without food. Dosing ...
-
WARNINGS User Safety Warnings - Not for use in humans. Keep this drug out of the reach of children. Wash hands immediately after handling tablets. In case of accidental ingestion, seek medical attention ...
-
PRECAUTIONS Dogs should be up to date on vaccinations prior to starting Zenrelia (see Target Animal Safety). The safe use of Zenrelia has not been evaluated in breeding, pregnant, or lactating dogs. Decreased ...
-
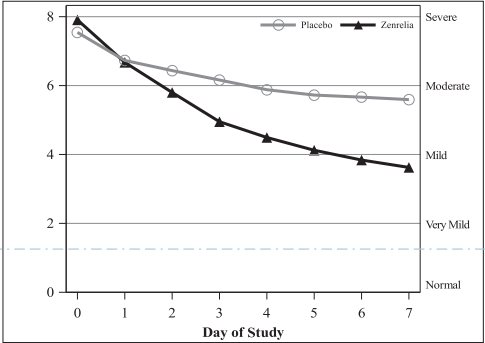
ADVERSE REACTIONS Control of Atopic Dermatitis - In a masked field study assessing effectiveness and safety of Zenrelia for the control of atopic dermatitis in dogs, 181 Zenrelia-treated dogs and 87 placebo-treated ...
-
CONTACT INFORMATION To report suspected adverse events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US Inc at 1-888-545-5973. For additional information about ...
-
CLINICAL PHARMACOLOGY Mechanism of Action - Ilunocitinib is a non-selective JAK inhibitor which inhibits the function of a variety of pruritogenic, pro-inflammatory and allergy related cytokines that are dependent upon ...
-
EFFECTIVENESS Control of Atopic Dermatitis: A masked, placebo-controlled field study was conducted at 25 veterinary clinics in the US and Canada, enrolling 268 client-owned dogs diagnosed with atopic ...
-
TARGET ANIMAL SAFETY Margin of Safety Study: Zenrelia was administered to 40 (8 dogs per group) healthy, 11 to 12-month-old, fed Beagle dogs, once daily at 0X, 1X, 2X, 3X and 5X the maximum exposure dose of 0.8 ...
-
STORAGE CONDITIONS Store at room temperature between 15 to 25°C (59 to 77°F), excursions permitted between 5 to 40°C (41 to 104°F).
-
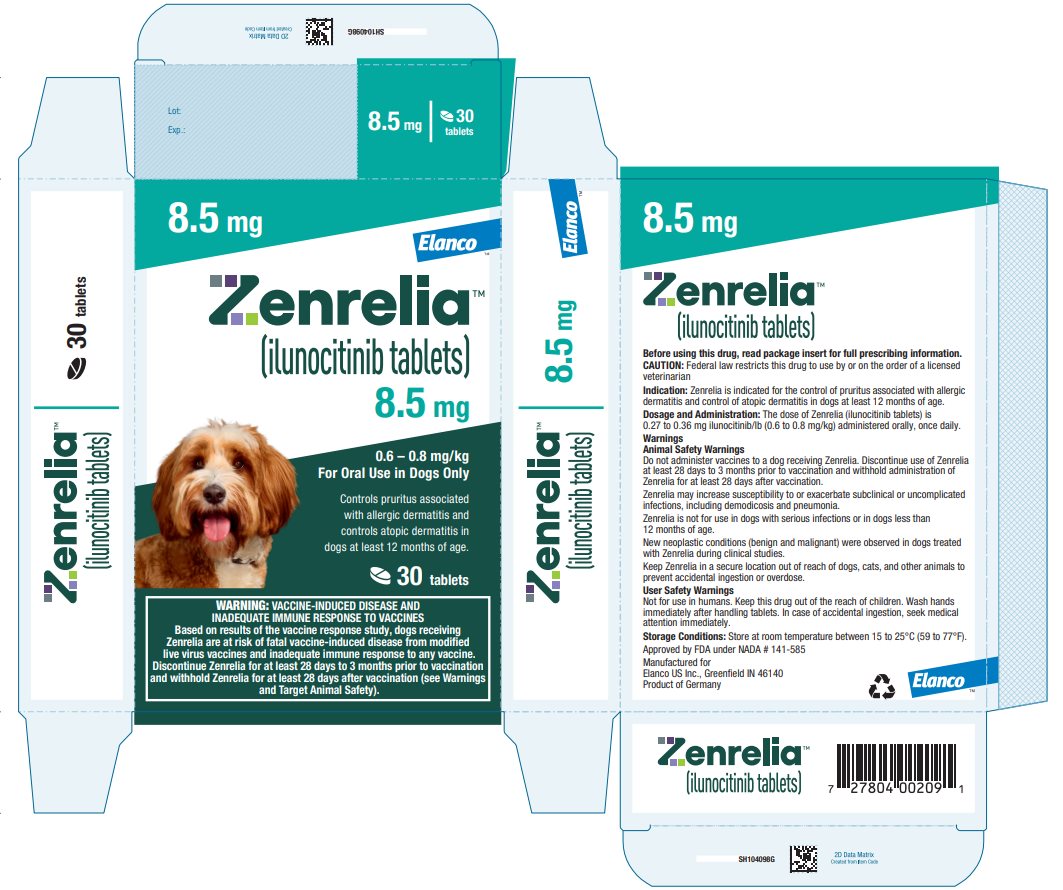
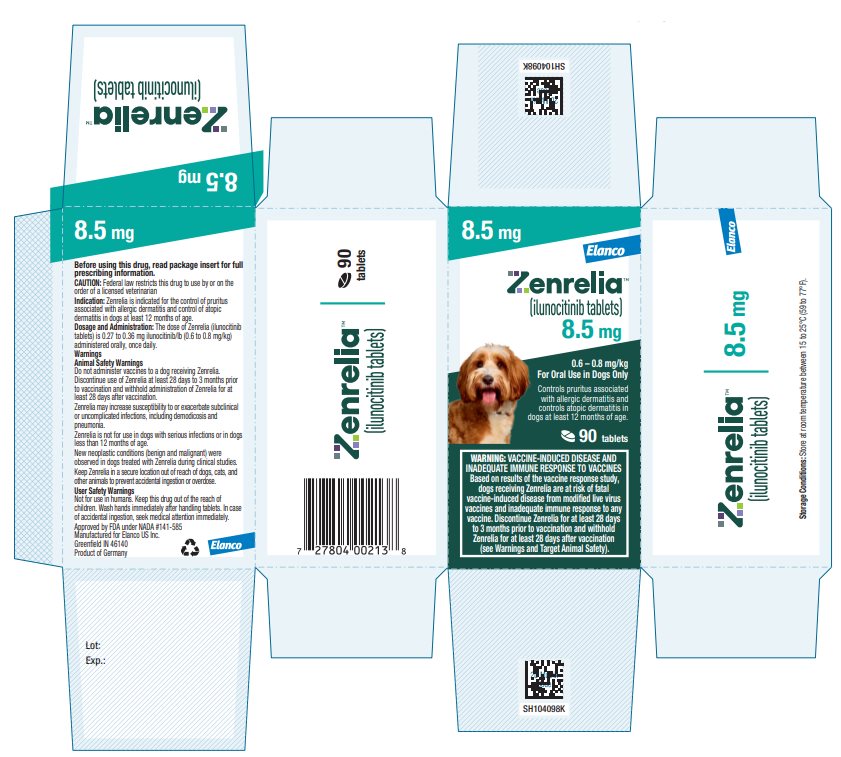
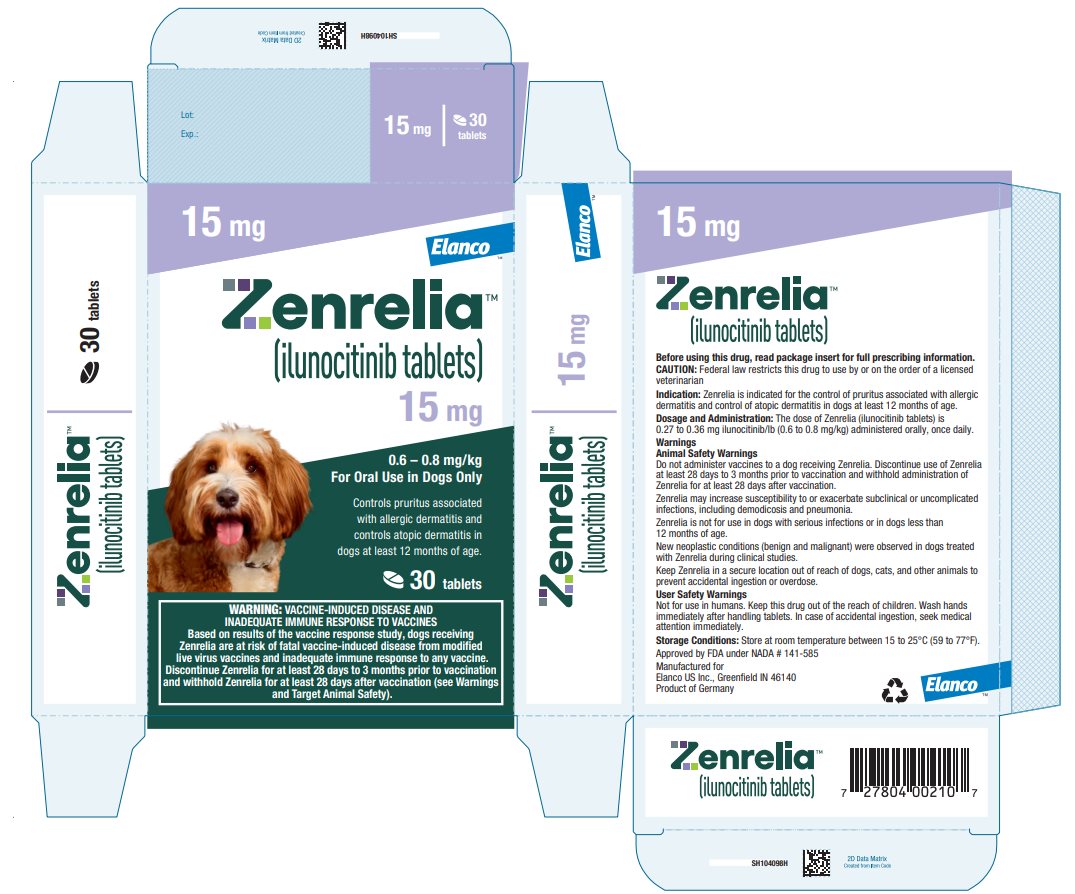
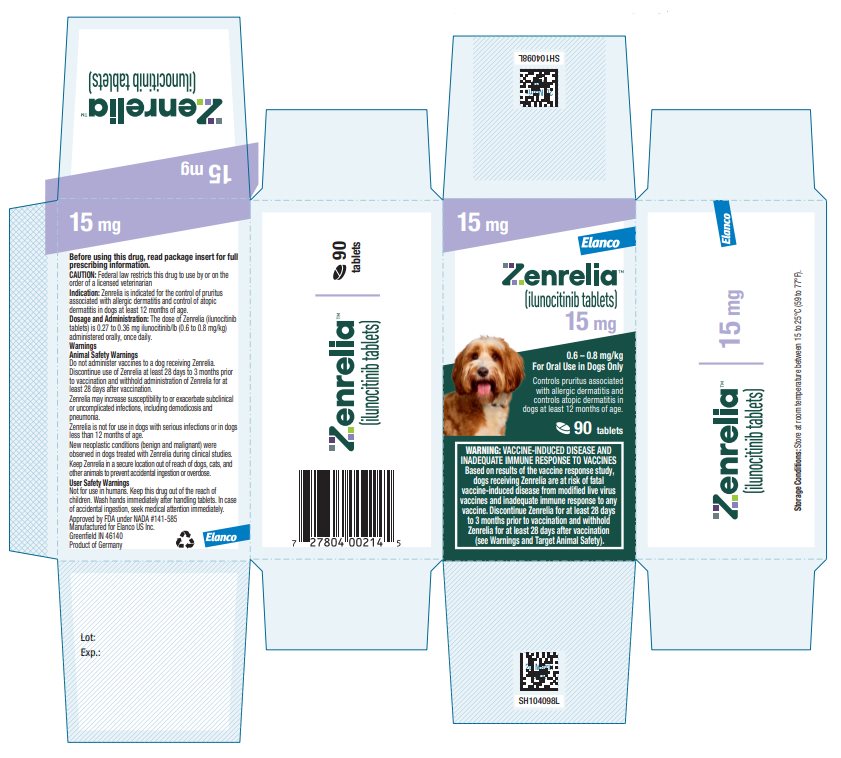
HOW SUPPLIED Zenrelia (ilunocitinib tablets) is available in scored tablets in four strengths: 4.8 mg, 6.4 mg, 8.5 mg, and 15 mg. Each tablet strength is available in 10 and 30 count blister packages and 90 ...
-
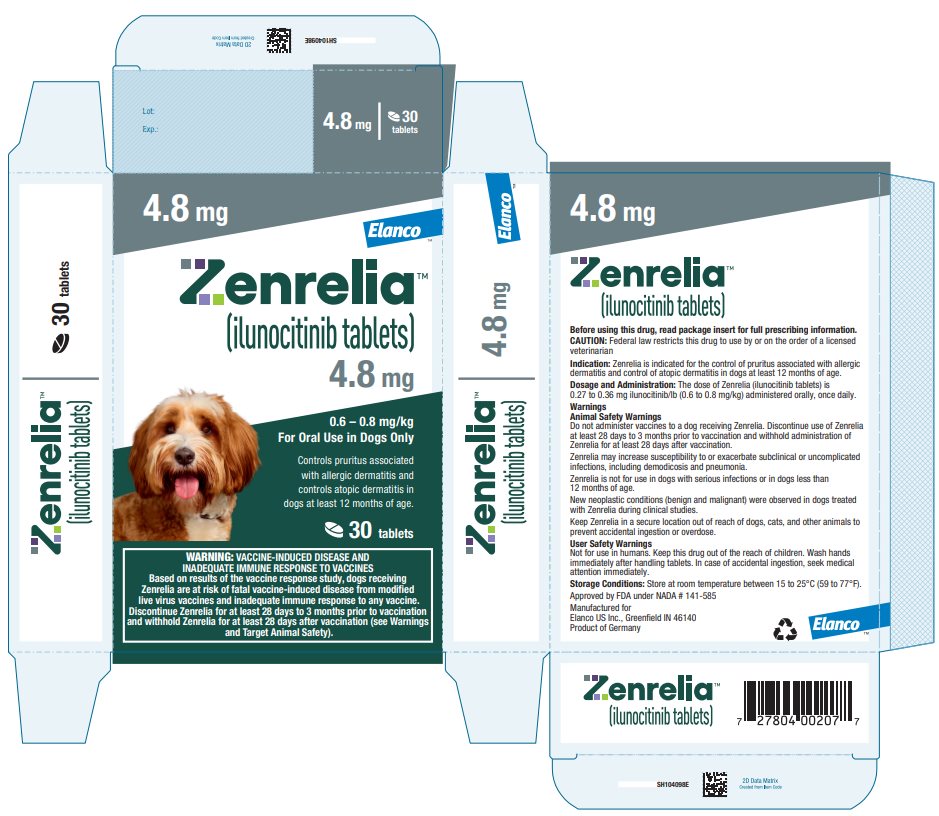
Principal Display Panel - Zenrelia 4.8 mg Elanco™ Zenrelia™ (ilunocitinib tablets) 4.8 mg - 0.6 – 0.8 mg/kg - For Oral Use in Dogs Only - Controls pruritus associated - with allergic dermatitis and - controls atopic dermatitis in - dogs at ...
-
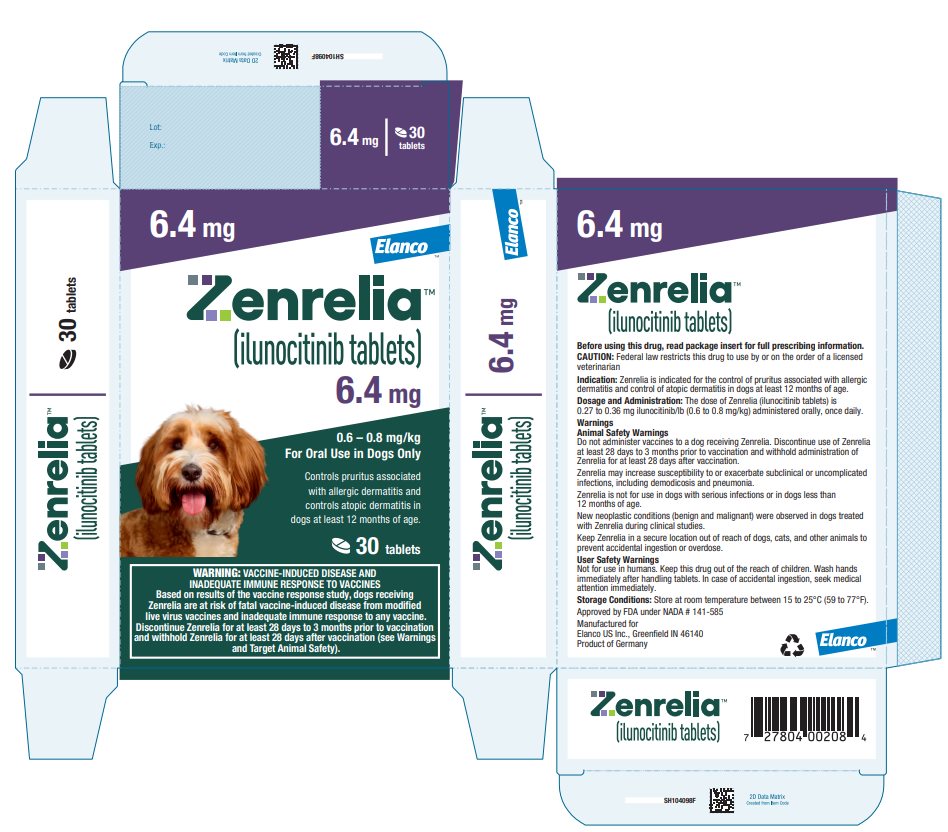
Principal Display Panel - Zenrelia 6.4 mg Elanco™ Zenrelia™ (ilunocitinib tablets) 6.4 mg - 0.6 – 0.8 mg/kg - For Oral Use in Dogs Only - Controls pruritus associated - with allergic dermatitis and - controls atopic dermatitis in - dogs at ...
-
Principal Display Panel - Zenrelia 8.5 mg Elanco™ Zenrelia™ (ilunocitinib tablets) 8.5 mg - 0.6 – 0.8 mg/kg - For Oral Use in Dogs Only - Controls pruritus associated - with allergic dermatitis and - controls atopic dermatitis in - dogs at ...
-
Principal Display Panel - Zenrelia 15 mg Elanco™ Zenrelia™ (ilunocitinib tablets) 15 mg - 0.6 – 0.8 mg/kg - For Oral Use in Dogs Only - Controls pruritus associated - with allergic dermatitis and - controls atopic dermatitis in - dogs at ...
-
INGREDIENTS AND APPEARANCEProduct Information