Label: IMPAVIDO- miltefosine capsule
- NDC Code(s): 69051-300-01
- Packager: Profounda, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IMPAVIDO safely and effectively. See full prescribing information for IMPAVIDO. IMPAVIDO (miltefosine) capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
- •
- Pregnancy: IMPAVIDO is contraindicated in pregnancy. Based on animal data, miltefosine may cause fetal harm [see Contraindications (4.1), Warnings and Precautions (5.1), and Use in Specific Populations (8.1)].
- •
- Females of Reproductive Potential: Verify pregnancy status prior to initiating IMPAVIDO. To prevent pregnancy, females of reproductive potential should use effective contraception during treatment and for 5 months after the last dose [see Dosage and Administration (2), Warnings and Precautions (5.1), Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
-
1 INDICATIONS AND USAGEIMPAVIDO (miltefosine) capsules are indicated in adults and pediatric patients 12 years of age and older weighing greater than or equal to 30 kg (66 lbs) for the treatment of: • Visceral ...
-
2 DOSAGE AND ADMINISTRATIONVerify pregnancy status prior to initiating IMPAVIDO in females of reproductive potential [see Use in Specific Populations, (8.3)]. The treatment duration is 28 consecutive days. Administer with ...
-
3 DOSAGE FORMS AND STRENGTHSIMPAVIDO® (miltefosine) oral capsules are opaque, red, hard gelatin capsules with “PLB” imprinted on the capsule body and “MILT 50” imprinted on the cap using a white ink. Each capsule contains ...
-
4 CONTRAINDICATIONS4.1 Pregnancy - IMPAVIDO is contraindicated in patients who are pregnant. Based on animal data, miltefosine may cause fetal harm. [see Boxed Warning, Warnings and Precautions (5.1) and Use in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - IMPAVIDO is contraindicated in patients who are pregnant. Based on animal data, miltefosine may cause fetal harm. Embryo-fetal toxicity, including death and fetal ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
7 DRUG INTERACTIONSIn vitro and animal metabolism studies showed that miltefosine did not markedly induce or inhibit the activity of the major human cytochrome P450 enzymes [see Clinical Pharmacology (12.3)]. The ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to IMPAVIDO during pregnancy. Healthcare providers are ...
-
10 OVERDOSAGEThe common adverse effects of vomiting, diarrhea, and abdominal pain are likely in case of overdose. Institute adequate hydration to prevent the risk of impaired renal function and replace ...
-
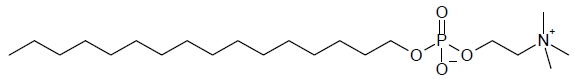
11 DESCRIPTIONIMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent. The chemical name of miltefosine is 2-[[(hexadecyloxy)hydroxyphosphenyl]oxy]-N,N,N-trimethylethylammonium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Miltefosine is an anti-leishmanial agent [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Cardiac Electrophysiology: The effect of IMPAVIDO on the QTc interval ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Mutagenicity/Carcinogenicity: Miltefosine tested negative in the AMES-Salmonella test, DNA-amplification test, chromosomal aberration ...
-
14 CLINICAL STUDIES14.1 Treatment of Visceral Leishmaniasis - One randomized, open-label, active-controlled study was conducted to evaluate the efficacy of IMPAVIDO in the treatment of visceral leishmaniasis in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach IMPAVIDO capsule contains 50 mg miltefosine in an opaque, red, hard gelatin capsule. IMPAVIDO capsules are supplied in a folded peel/push-through child-resistant blister card. Each blister ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) Dosing Instructions - • IMPAVIDO is administered with food to ameliorate ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised 3/2025 - MEDICATION GUIDE - IMPAVIDO® (Im-PA-vee-do) (miltefosine) capsules, for oral ...
-
PRINCIPAL DISPLAY PANEL - Carton Carton - NDC 69051-300-01 28 Capsules - Impavido® (miltefosine) capsules - 50 mg per Capsule - Rx only PROFOUNDA® Contains 2 blister cards, 14 blisters per card, 1 capsule per ...
-
INGREDIENTS AND APPEARANCEProduct Information