Label: SKYSONA- elivaldogene autotemcel suspension
- NDC Code(s): 73554-2111-1
- Packager: bluebird bio, Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SKYSONA safely and effectively. See full prescribing information for SKYSONA.
SKYSONA® (elivaldogene autotemcel) suspension for intravenous infusion
Initial U.S. Approval: 2022WARNING: HEMATOLOGIC MALIGNANCY
See full prescribing information for complete boxed warning.
Hematologic malignancies, including life-threatening cases of myelodysplastic syndrome and acute myeloid leukemia, have occurred in patients treated with SKYSONA. The cancers appear to be the result of the SKYSONA lentiviral vector, Lenti-D, integration in proto-oncogenes. Monitor patients closely for evidence of malignancy through complete blood counts at least every 3 months and through assessments for evidence for clonal expansion or predominance at least twice in the first year and annually thereafter; consider bone marrow evaluations as clinically indicated. (5.1)
RECENT MAJOR CHANGES
Boxed Warning 4/2024 Warnings and Precautions, Hematologic Malignancy (5.1) 4/2024 Warnings and Precautions, Serious Infections (5.2) 4/2024 Warnings and Precautions, Prolonged Cytopenias (5.3) 4/2024 INDICATIONS AND USAGE
SKYSONA is an autologous hematopoietic stem cell-based gene therapy indicated to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD). Early, active CALD refers to asymptomatic or mildly symptomatic (neurologic function score, NFS ≤ 1) boys who have gadolinium enhancement on brain magnetic resonance imaging (MRI) and Loes scores of 0.5-9.
This indication is approved under accelerated approval based on 24-month Major Functional Disability (MFD)-free survival. [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
Limitations of Use
- SKYSONA does not treat or prevent adrenal insufficiency. (1)
- An immune response to SKYSONA may cause rapid loss of efficacy of SKYSONA in patients with full deletions of the human adenosine triphosphate binding cassette, sub family D, member 1 (ABCD1) gene. (1)
- SKYSONA has not been studied in CALD secondary to head trauma. (1)
- Given the risk of hematologic malignancy with SKYSONA, and unclear long-term durability of SKYSONA and human adrenoleukodystrophy protein (ALDP) expression, careful consideration should be given to the timing of treatment for each boy and treatment of boys with isolated pyramidal tract disease as clinical manifestations do not usually occur until adulthood. (1)
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
- Patients must undergo hematopoietic stem cell (HSC) mobilization and apheresis to obtain CD34+ cells for SKYSONA manufacturing. (2.2)
- Dosing of SKYSONA is based on the number of CD34+ cells in the infusion bag(s) per kg of body weight. (2.1)
- The minimum recommended dose is 5.0 × 106 CD34+ cells/kg. (2.1)
- Full myeloablative and lymphodepleting conditioning must be administered before infusion of SKYSONA. (2.2)
- Verify the patient's identity matches the unique patient identification information on the SKYSONA infusion bag(s) prior to infusion. (2.2)
- Do not sample, alter, or irradiate SKYSONA. (2.2)
- Do not use an in-line blood filter or an infusion pump. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Life-threatening bacterial and viral infections may occur. Monitor patients for signs and symptoms of infection. (5.2)
- Prolonged Cytopenias: Patients may exhibit cytopenias >1 year after treatment. Monitor patients for bleeding and infection. (5.3)
- Delayed Platelet Engraftment: Monitor patients for thrombocytopenia and bleeding until platelet engraftment and count recovery. (5.4)
- Risk of Neutrophil Engraftment Failure: Monitor absolute neutrophil counts and if neutrophil engraftment does not occur, give rescue cells. (5.5)
ADVERSE REACTIONS
- Most common non-laboratory adverse reactions (≥ 20%): mucositis, nausea, vomiting, febrile neutropenia, alopecia, decreased appetite, abdominal pain, constipation, pyrexia, diarrhea, headache, rash. (6.1)
- Most common Grade 3 or 4 laboratory abnormalities (≥ 40%): leukopenia, lymphopenia, thrombocytopenia, neutropenia, anemia, hypokalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact bluebird bio at 1-833-999-6378 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Anti-retrovirals: Do not take these medications from at least one month prior to starting mobilization agents until all cycles of apheresis are complete and after the expected duration of elimination. (7.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEMATOLOGIC MALIGNANCY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation Before SKYSONA Infusion
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Malignancy
5.2 Serious Infections
5.3 Prolonged Cytopenias
5.4 Delayed Platelet Engraftment
5.5 Risk of Neutrophil Engraftment Failure
5.6 Hypersensitivity Reactions
5.7 Anti-retroviral Use
5.8 Laboratory Test Interference
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Vaccines
7.2 Anti-retrovirals
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.6 Patients with a Full ABCD1 Gene Deletion
8.7 Renal Impairment
8.8 Hepatic Impairment
8.9 Patients Seropositive for Human Immunodeficiency Virus (HIV)
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HEMATOLOGIC MALIGNANCY
Hematologic malignancies, including life-threatening cases of myelodysplastic syndrome and acute myeloid leukemia, have occurred in patients treated with SKYSONA. Patients have been diagnosed between 14 months and 7.5 years after SKYSONA administration, and the cancers appear to be the result of the SKYSONA lentiviral vector, Lenti-D, integration in proto-oncogenes. Monitor patients closely for evidence of malignancy through complete blood counts at least every 3 months. Monitor patients through assessments for evidence for clonal expansion or predominance at least twice in the first year and annually thereafter; consider bone marrow evaluations as clinically indicated [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
SKYSONA is indicated to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD). Early, active cerebral adrenoleukodystrophy refers to asymptomatic or mildly symptomatic (neurologic function score, NFS ≤ 1) boys who have gadolinium enhancement on brain magnetic resonance imaging (MRI) and Loes scores of 0.5-9.
This indication is approved under accelerated approval based on 24-month Major Functional Disability (MFD)-free survival [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Limitations of Use
SKYSONA does not prevent the development of or treat adrenal insufficiency due to adrenoleukodystrophy.
An immune response to SKYSONA may limit the persistence of descendent cells of SKYSONA, causing rapid loss of efficacy of SKYSONA in patients with full deletions of the human adenosine triphosphate binding cassette, sub family D, member 1 (ABCD1) gene.
SKYSONA has not been studied in patients with CALD secondary to head trauma.
Given the risk of hematologic malignancy with SKYSONA, and unclear long-term durability of SKYSONA and human adrenoleukodystrophy protein (ALDP) expression, careful consideration should be given to the appropriateness and timing of treatment for each boy, especially for boys with isolated pyramidal tract disease based on available treatment options since their clinical symptoms do not usually occur until adulthood.
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
2.1 Dose
SKYSONA is provided as a single dose for infusion containing a suspension of CD34+ cells in one or two infusion bags. The minimum recommended dose of SKYSONA is 5.0 × 106 CD34+ cells/kg.
The dose is calculated based on the patient's weight prior to first apheresis. See the Lot Information Sheet provided with the product shipment for additional information pertaining to dose.
2.2 Preparation Before SKYSONA Infusion
Before mobilization, apheresis, and conditioning are initiated, confirm that hematopoietic stem cell (HSC) transplantation is appropriate for the patient.
Perform screening for hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus 1 & 2 (HIV-1/HIV-2) and Human T-lymphotropic virus 1 & 2 (HTLV-1/HTLV-2) in accordance with clinical guidelines before collection of cells for manufacturing.
Mobilization and Apheresis
Patients are required to undergo HSC mobilization followed by apheresis to obtain CD34+ cells for product manufacturing. Weigh the patient prior to the first apheresis collection. Collect a minimum number of CD34+ cells of 12 × 106 CD34+ cells/kg.
A back-up collection of CD34+ cells of ≥ 1.5 × 106 CD34+ cells/kg (if collected by apheresis) or ≥ 1.0 × 108 TNC/kg (Total Nucleated Cells, if collected by bone marrow harvest) is required. Collect and cryopreserve these cells prior to initiating conditioning and infusion with SKYSONA. The back-up collection may be needed for rescue treatment if there is: 1) compromise of SKYSONA after initiation of conditioning and before SKYSONA infusion, 2) primary engraftment failure, or 3) loss of engraftment after infusion with SKYSONA.
Myeloablative and Lymphodepleting Conditioning
Full myeloablative and lymphodepleting conditioning must be administered before infusion of SKYSONA. Consult prescribing information for the conditioning agents prior to treatment.
Do not begin conditioning until SKYSONA has been received and stored at the treatment center and the availability of the back-up collection of CD34+ cells is confirmed. After completion of conditioning, allow a minimum of 48 hours of washout before SKYSONA infusion.
Receipt and Storage of SKYSONA
- Ensure the availability of vapor phase of liquid nitrogen storage at less than or equal to -140°C (-220°F) at the treatment center.
- SKYSONA is shipped to the treatment center in the vapor phase of liquid nitrogen shipper.
- Confirm Patient Identifiers on the product labels and Lot Information Sheet within the shipper.
- If there are any concerns about the product or packaging upon receipt, contact bluebird bio at 1-833-999-6378.
- Keep the infusion bag(s) in the metal cassette(s) and transfer SKYSONA from the vapor phase of liquid nitrogen shipper to the treatment center vapor phase of liquid nitrogen storage at less than or equal to -140°C (-220°F) until ready for thaw and administration.
Preparation of SKYSONA for Infusion
Coordinate the timing of SKYSONA thaw and infusion. Confirm the infusion time in advance and adjust the start time of SKYSONA thaw such that it will be available for infusion when the patient and healthcare providers are ready.
SKYSONA contains human blood cells that are genetically modified with replication-incompetent, self-inactivating Lenti-D lentiviral vector (LVV). Follow universal precautions and local biosafety guidelines for handling and disposal of SKYSONA to avoid potential transmission of infectious diseases.
- Remove each metal cassette from liquid nitrogen storage.
- Confirm that SKYSONA is printed on the infusion bag(s) label(s).
- Confirm that patient identity matches the unique patient identification information located on the SKYSONA infusion bag(s). Do not infuse SKYSONA if the information on the patient-specific label on the infusion bag does not match the intended patient, and contact bluebird bio at 1-833-999-6378.
- A SKYSONA dose may be contained in one or two patient-specific infusion bags. Ensure that the correct number of infusion bags are present. Use the accompanying Lot Information Sheet to confirm that each infusion bag is within the expiration date prior to preparation of SKYSONA for infusion.
- Remove the overwrap and inspect each infusion bag for any breaches of integrity before thawing and infusion. If an infusion bag is compromised, follow the local guidelines and contact bluebird bio immediately at 1-833-999-6378.
- If more than one infusion bag is provided to achieve the treatment dose, thaw and administer each infusion bag completely before proceeding to thaw the next infusion bag. Maintain the second infusion bag, if applicable, within the cold-storage dewar to maintain temperature less than or equal to -140°C (-220°F) until time to thaw.
- Thaw SKYSONA at 37°C (98.6°F) in a water bath or dry bath. Thawing of each infusion bag takes approximately 2 to 4 minutes. Promptly remove the infusion bag from the bath once thawed. Do not leave SKYSONA unattended and do not submerge the infusion ports if thawed in a water bath.
- After thawing, mix SKYSONA gently by massaging the infusion bag to disperse clumps of cellular material until all of the contents are uniform. If visible cell clumps remain, continue to gently mix the contents of the bag. Most small clumps of cellular material should disperse with gentle manual mixing. Do not filter, wash, spin down and/or resuspend SKYSONA in new media prior to infusion.
- Do not sample, alter, irradiate or refreeze SKYSONA.
2.3 Administration
SKYSONA is for autologous use only. The patient's identity must match the patient identifiers on the SKYSONA cassette(s) and infusion bag(s). Do not infuse SKYSONA if the information on the patient-specific label does not match the intended patient.
- Do not use an in-line blood filter or an infusion pump.
- Before infusion, confirm that the patient's identity matches the unique patient identifiers on the SKYSONA infusion bag(s). The total number of infusion bags to be administered should also be confirmed with the Lot Information Sheet.
- Prime the tubing of the infusion set with 0.9% sodium chloride solution prior to infusion.
- Expose the sterile port on the infusion bag by tearing off the protective wrap covering the port.
- Access the SKYSONA infusion bag and infuse per the treatment center's standard procedures for administration of cell therapy products.
- Complete the infusion of SKYSONA as soon as possible, and no more than 4 hours after thawing.
- Administer each infusion bag of SKYSONA via intravenous infusion (drip) by gravity flow over a period of less than 60 minutes.
- After the entire content of the infusion bag is infused, flush all SKYSONA remaining in the infusion bag and any associated tubing with at least 50 mL of 0.9% sodium chloride solution to ensure that as many cells as possible are infused into the patient.
- If more than one infusion bag is provided, administer each infusion bag completely before proceeding to thaw (following Section 2.2 steps 7-8) and infuse (following Section 2.3 steps 2-6) the next infusion bag.
After SKYSONA Administration
Standard procedures for patient management after HSC transplantation should be followed after SKYSONA infusion.
- Irradiate any blood products required within the first 3 months after SKYSONA infusion.
- Patients treated with SKYSONA should not donate blood, organs, tissues, or cells at any time in the future.
-
3 DOSAGE FORMS AND STRENGTHS
SKYSONA is a cell suspension for intravenous infusion.
SKYSONA is composed of one or two infusion bags which contain 4 to 30 × 106 cells/mL suspended in cryopreservation solution [see How Supplied/Storage and Handling (16)]. Each infusion bag contains approximately 20 mL of SKYSONA. A single dose of SKYSONA contains a minimum of 5.0 × 106 CD34+ cells per kg of body weight, suspended in cryopreservation solution.
See the Lot Information Sheet for actual dose.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Malignancy
Hematologic malignancies, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), have developed in patients treated with SKYSONA in clinical studies between 14 months and 7.5 years after SKYSONA administration [see Adverse Reactions (6.1)]. Malignancies are life-threatening and death related to treatment for malignancy has occurred.
SKYSONA Lenti-D lentiviral vector integration into proto-oncogenes, including MECOM, appears to have mediated the cases of hematologic malignancy. All patients treated with SKYSONA in clinical studies have integrations into MECOM; it is unknown which integrations into MECOM or other proto-oncogenes are likely to lead to malignancy.
Because of the risk of hematologic malignancy, carefully consider alternative therapies including allogeneic hematopoietic stem cell transplant for patients who have a suitable, willing, and available matched sibling donor, prior to the decision to treat a child with SKYSONA.
Consider consultation with hematology experts prior to SKYSONA treatment to inform benefit-risk treatment decision and to ensure adequate monitoring for hematologic malignancy. Consider performing the following baseline hematologic assessments: complete blood count with differential, hematopathology review of peripheral blood smear, and bone marrow biopsy (core and aspirate) with flow cytometry, conventional karyotyping, and next generation sequencing (NGS) with a molecular panel appropriate for age and including coverage for gene mutations expected in myeloid and lymphoid malignancies; and testing for germline mutations that are associated with hematologic malignancy.
Early diagnosis of hematologic malignancy can be critically important, therefore, monitor patients treated with SKYSONA lifelong for hematologic malignancy. For at least the first fifteen years after treatment with SKYSONA, monitor via complete blood count (with differential) at least every 3 months and via integration site analysis or other testing for evidence of clonal expansion and predominance at least twice in the first year and then annually. Consider appropriate expert consultation and additional testing such as more frequent complete blood count (with differential) and integration site analysis, bone marrow studies, and gene expression studies in the following settings after treatment with SKYSONA:
- Delayed or failed engraftment of platelets or other cell lines (while all patients are at risk for hematologic malignancy, patients who do not achieve unsupported platelet counts of ≥ 20 × 109/L on or after Day 60 appear to be at higher risk); or
- New or prolonged cytopenias; or,
- Presence of clonal expansion or predominance (e.g., increasing relative frequency of an integration site, especially if ≥ 10% and present in MECOM or another proto-oncogene known to be involved in hematologic malignancy).
If hematologic malignancy is detected in a patient who received SKYSONA, contact bluebird bio at 1-833-999-6378 for reporting and to obtain instructions on collection of samples for further testing.
Post-Marketing Long Term Follow-Up Study
Patients who intend to receive treatment with SKYSONA are encouraged to enroll in the study, as available, to assess the long-term safety of SKYSONA and the risk of malignancies occurring after treatment with SKYSONA by calling bluebird bio, Inc. at 1-833-999-6378. The study includes monitoring (at pre-specified intervals) for clonal expansion.
5.2 Serious Infections
Severe infections, including life-threatening and fatal infections, have occurred in patients after SKYSONA infusion.
Febrile neutropenia was commonly observed in clinical studies and may be a sign of a serious infection. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
Monitor patients for signs and symptoms of infection before and after SKYSONA administration and treat appropriately. Administer prophylactic antimicrobials according to best clinical practices and clinical guidelines.
Avoid administration of SKYSONA in patients with active infections.
5.3 Prolonged Cytopenias
Patients may exhibit cytopenias, including pancytopenia, for > 1 year following conditioning and SKYSONA infusion [see Adverse Reactions (6.1)].
Monitor blood counts until normalization and assess patients for signs and symptoms of bleeding and/or infection prior to and after SKYSONA administration.
5.4 Delayed Platelet Engraftment
Delayed platelet engraftment (platelet count ≤ 50 × 109/L beyond 60 days after treatment with SKYSONA) has been observed [see Adverse Reactions (6.1)]. Bleeding risk is increased prior to platelet engraftment and may continue after engraftment in patients with prolonged thrombocytopenia.
Patients should be made aware of the risk of bleeding until platelet recovery has been achieved. Monitor patients for thrombocytopenia and bleeding according to standard guidelines. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
5.5 Risk of Neutrophil Engraftment Failure
There is a potential risk of neutrophil engraftment failure after treatment with SKYSONA. Neutrophil engraftment failure was defined as failure to achieve 3 consecutive absolute neutrophil counts (ANC) ≥ 0.5 × 109 cells/L obtained on different days by Day 43 after infusion of SKYSONA. Monitor neutrophil counts until engraftment has been achieved. If neutrophil engraftment failure occurs in a patient treated with SKYSONA, provide rescue treatment with the back-up collection of CD34+ cells [see Adverse Reactions (6.1)].
5.6 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of SKYSONA. The dimethyl sulfoxide (DMSO) in SKYSONA may cause hypersensitivity reactions, including anaphylaxis which is potentially life-threatening and requires immediate intervention.
5.7 Anti-retroviral Use
Patients should not take anti-retroviral medications for at least one month prior to mobilization or the expected duration for elimination of the medications, and until all cycles of apheresis are completed. Anti-retroviral medications may interfere with manufacturing of the apheresed cells [see Drug Interactions (7.2)].
If a patient requires anti-retrovirals for HIV prophylaxis, mobilization and apheresis of CD34+ cells should be delayed until HIV infection is adequately ruled out.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hematologic Malignancy [see Warnings and Precautions (5.1)]
- Serious Infections [see Warnings and Precautions (5.2)]
- Prolonged Cytopenias [see Warnings and Precautions (5.3)]
- Delayed Platelet Engraftment [see Warnings and Precautions (5.4)]
- Risk of Neutrophil Engraftment Failure [see Warnings and Precautions (5.5)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflect exposure to a single dose of SKYSONA in 67 patients with CALD. Data were obtained from two single-arm trials and, for 36 patients, from a long-term follow-up study [see Clinical Studies (14)]. The median (min, max) age across studies was 6 (4, 14) years; 100% were male; 54% were White/Caucasian, 4% were Black or African American, 1% were Asian, 10% were of other races including mixed race, and 30% did not report race; 25% were of Hispanic ethnicity. The median (min, max) duration of follow-up at time of initial approval was 24 (1, 88) months.
In the two trials, serious adverse reactions from Day 1 (SKYSONA infusion) to last follow-up occurred in 54% of patients. The most common non-laboratory, serious adverse reactions (≥ 3% incidence) that occurred after treatment with SKYSONA were febrile neutropenia (18%), pyrexia (fever) (18%), seizure (7%), myelodysplastic syndrome (4%), pseudomonal bacteremia (3%), pancytopenia (3%), vascular device infection (3%), mucositis (3%), and vomiting (3%).
Most common non-laboratory adverse reactions by time of onset follow:
- During mobilization and conditioning and occurring in ≥ 20% of patients: nausea (79%), vomiting (72%), decreased appetite (42%), catheter site pain (39%), constipation (30%), headache (24%), abdominal pain (21%), rash (13%)
- In the first 60 days after treatment with SKYSONA in ≥ 15% of patients: mucositis (88%), febrile neutropenia (73%), alopecia (72%), abdominal pain (33%), vomiting (31%), decreased appetite (31%), pyrexia (27%), nausea (27%), constipation (21%), diarrhea (21%), epistaxis (19%), pruritus (18%), headache (16%), oropharyngeal pain (16%), skin hyperpigmentation (16%), anxiety (15%)
- Between 60 days and 1 year after treatment with SKYSONA in ≥ 5% of patients: pyrexia (fever) (9%) and vomiting (6%)
Table 1 presents non-laboratory adverse reactions reported in at least 10% of patients between the start of conditioning and 24 months after SKYSONA administration. Table 2 presents Grade 3 or 4 laboratory abnormalities that occurred in at least 40% of patients between the start of conditioning and 24 months after SKYSONA administration.
Table 1: Non-Laboratory Adverse Reactions Reported in ≥ 10% of Patients Between the Start of Conditioning and 24 Months Following Treatment with SKYSONA* Adverse Reaction Any Grade
n (%)Grade 3 or Higher
n (%)- *
- Includes adverse events associated with conditioning.
- †
- Febrile neutropenia includes febrile bone marrow aplasia and febrile neutropenia.
- ‡
- Tachycardia includes sinus tachycardia and tachycardia.
- §
- Mucositis includes anal inflammation, colitis, gastrointestinal inflammation, mucosal inflammation, proctitis, and stomatitis.
- ¶
- Encompasses more than one system organ class.
- #
- Abdominal pain includes abdominal discomfort, abdominal pain, and abdominal pain upper.
- Þ
- Transfusion reaction includes allergic transfusion reaction and anaphylactic transfusion reaction.
- ß
- Anxiety includes akathisia, agitation, anxiety, and irritability.
- à
- Oropharyngeal pain includes mouth ulceration, oral pain, and oropharyngeal pain.
- è
- Rash includes rash, rash erythematous, rash maculo-papular, and urticaria.
- ð
- Pruritus includes anal pruritus, pruritus, and pruritus allergic.
Blood and lymphatic system disorders -- -- Febrile neutropenia† 49 (73%) 49 (73%) Cardiac disorders -- -- Tachycardia‡ 10 (15%) 0 Eye disorders -- -- Vision blurred 7 (10%) 0 Gastrointestinal disorders -- -- Mucositis§¶ 62 (92%) 34 (51%) Nausea 56 (84%) 17 (25%) Vomiting 51 (76%) 12 (18%) Abdominal pain# 30 (45%) 2 (3%) Constipation 28 (42%) 0 Diarrhea 19 (28%) 1 (1%) General disorders and administration site conditions -- -- Pyrexia 24 (36%) 3 (4%) Injury, poisoning and procedural complications -- -- Transfusion reactionÞ 8 (12%) 2 (3%) Metabolism and nutrition disorders -- -- Decreased appetite 43 (64%) 27 (40%) Nervous system disorders -- -- Headache 19 (28%) 0 Anxiety߶ 10 (15%) 0 Respiratory, thoracic, and mediastinal disorders -- -- Epistaxis 13 (19%) 5 (7%) Oropharyngeal painච12 (18%) 3 (4%) Cough 7 (10%) 0 Skin and subcutaneous tissue disorders -- -- Alopecia 48 (72%) 1 (1%) Rashè 14 (21%) 0 Pruritusð¶ 13 (19%) 0 Skin hyperpigmentation 12 (18%) 0 Vascular disorders -- -- Hypertension 8 (12%) 1 (1%) Hematologic Malignancy
As of April 2024, hematologic malignancies have been diagnosed in 6/67 (9%) clinical study patients. At diagnosis, all patients had high-frequency integrations in oncogenes; most of which were in MECOM. Pathological diagnoses ranged between myelodysplastic syndrome (MDS)-unilineage dysplasia to acute myeloid leukemia. Most patients required chemotherapy with or without allogeneic hematopoietic stem cell transplant.
Serious Infections
Important opportunistic infections that have been diagnosed within the first 3 months after treatment with SKYSONA include BK cystitis, cytomegalovirus reactivation, human herpesvirus-6 viremia, candidiasis, and bacteremias. Opportunistic infections after the first 3 months include an atypical mycobacterium vascular device infection, pseudomonas bacteremia, and Epstein-Barr virus reactivations diagnosed as late as 18 months after treatment with SKYSONA. Serious infections involving adenovirus include a case of transverse myelitis at 6 months that was attributed to adenovirus and entero/rhinovirus infection, and a fatal adenovirus infection at 21 months in a patient with CALD progression who developed multisystem organ failure.
Grade 3 or higher infections occurred in 21% of all patients (12% bacterial, 3% viral, and 6% unspecified). The most common Grade 3 or higher infections were vascular device infections (7% of patients) diagnosed as late as 6 months after treatment with SKYSONA, and bacteremias (6% of patients) diagnosed as late as 8 months after treatment with SKYSONA.
Table 2: Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 40% of Patients Between the Start of Conditioning and 24 Months Following Treatment with SKYSONA* Laboratory Abnormality Grade 3 or 4
n (%)- *
- Includes laboratory abnormalities associated with conditioning.
Leukopenia 67 (100%) Lymphopenia 67 (100%) Thrombocytopenia 67 (100%) Neutropenia 64 (96%) Anemia 56 (84%) Hypokalemia 28 (42%) Cytopenias
Grade 3 or higher cytopenias on or after Day 60 following SKYSONA infusion occurred in 47% of patients and included low platelet count (14%), low neutrophil count (22%), low lymphocyte count (27%), and low hemoglobin (2%). Grade 3 cytopenias persisted beyond Day 100 in 15% of patients and included low platelet count (7%), low neutrophil count (9%), and low lymphocyte count (6%).
Serious adverse reactions of pancytopenia occurred in two patients who required support with blood and platelet transfusions as well as growth factors (G-CSF for up to 6 months and eltrombopag for up to 14 months) after SKYSONA administration. One patient had intercurrent parvovirus infection and his pancytopenia was ongoing at least two years after SKYSONA administration. Pancytopenia in the other patient was ongoing until he was diagnosed with myelodysplastic syndrome approximately two years after SKYSONA administration.
Platelet Engraftment Delay
Platelet engraftment was defined as 3 consecutive platelet values ≥ 20 × 109/L on different days and no platelet transfusions administered for 7 days immediately preceding and during the evaluation period. Platelet engraftment was not achieved by Day 43 after SKYSONA administration in 13 of 63 patients (21%). Patients treated with SKYSONA achieved platelet engraftment at median (min, max) Day 29 (14, 108) in clinical studies, including two patients treated with a thrombopoietin receptor agonist at the time engraftment criteria were met until 10 or 14 months after treatment with SKYSONA. One of the two had persistence of mild thrombocytopenia after discontinuation of the eltrombopag, and the other remained severely thrombocytopenic (platelet count < 50 × 109/L) until he was diagnosed with myelodysplastic syndrome approximately 2 years after SKYSONA administration [see Warnings and Precautions (5.4)].
Neutrophil Engraftment
Neutrophil engraftment was defined as achieving 3 consecutive absolute neutrophil counts (ANC) ≥ 0.5 × 109 cells/L (after initial post-infusion nadir) obtained on different days by Day 43 after SKYSONA infusion. While all patients met criteria for neutrophil engraftment following treatment with SKYSONA in clinical trials, 7 of 67 patients (10%) required G-CSF beyond Day 43, including 3 patients who required G-CSF more than 3 months after treatment with SKYSONA. In three other patients, G-CSF discontinuation was followed by a decrease in neutrophil count to < 0.5 × 109 cells/L occurring within 3 days and lasting for two to five weeks [see Warnings and Precautions (5.5)].
-
7 DRUG INTERACTIONS
7.1 Vaccines
The safety and effectiveness of vaccination during or following SKYSONA treatment have not been studied. Vaccination is not recommended during the 6 weeks preceding the start of myeloablative conditioning, and until hematological recovery following treatment with SKYSONA. Where feasible, administer childhood vaccinations prior to myeloablative conditioning for SKYSONA.
7.2 Anti-retrovirals
Patients should not take anti-retroviral medications for at least one month prior to initiating medications for stem cell mobilization and for the expected duration for elimination of the medications, and until all cycles of apheresis are completed [see Warnings and Precautions (5.5)].
Anti-retroviral medications may interfere with SKYSONA manufacture.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with SKYSONA administration in pregnant women. Consider the risks associated with mobilization and conditioning agents on pregnancy and fertility.
No animal reproductive and developmental toxicity studies have been conducted to assess whether SKYSONA can cause fetal harm when administered to a pregnant woman.
No nonclinical germline transmission studies have been conducted with SKYSONA.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.3 Females and Males of Reproductive Potential
Contraception
Consult the Prescribing Information of the mobilization and conditioning agents for information on the need for effective contraception.
There are insufficient exposure data to provide a precise recommendation on duration of contraception following treatment with SKYSONA. Males capable of fathering a child and their female partners of childbearing potential should use an effective method of contraception (intra-uterine device or combination of hormonal and barrier contraception) from start of mobilization through at least 6 months after administration of SKYSONA.
8.4 Pediatric Use
The safety and efficacy of SKYSONA in children less than 4 years of age have not been established. No data are available [see Clinical Studies (14)].
8.6 Patients with a Full ABCD1 Gene Deletion
In the only patient in the SKYSONA clinical studies who had a full ABCD1 deletion, disease progression occurred. The patient experienced radiologic disease progression in the setting of declining peripheral blood vector copy number, suggesting loss of product efficacy which may have been immune mediated. The patient was subsequently treated with allogeneic hematopoietic stem cell transplant.
8.7 Renal Impairment
SKYSONA has not been studied in patients with renal impairment. Patients should be assessed for renal impairment to ensure hematopoietic stem cell (HSC) transplantation is appropriate.
8.8 Hepatic Impairment
SKYSONA has not been studied in patients with hepatic impairment. Patients should be assessed for hepatic impairment to ensure HSC transplantation is appropriate.
8.9 Patients Seropositive for Human Immunodeficiency Virus (HIV)
SKYSONA has not been studied in patients with HIV-1, HIV-2, HTLV-1, or HTLV-2. A negative serology test for HIV is necessary to ensure acceptance of apheresis material for SKYSONA manufacturing. Apheresis material from patients with a positive test for HIV will not be accepted for SKYSONA manufacturing.
-
11 DESCRIPTION
SKYSONA (elivaldogene autotemcel) is an autologous HSC-based gene therapy prepared from the patient's HSCs, which are collected via apheresis procedure(s). The autologous cells are enriched for CD34+ cells, then transduced ex vivo with Lenti-D LVV, and cultured with growth factors overnight. Lenti-D LVV is a replication-incompetent, self-inactivating LVV carrying ABCD1 cDNA that encodes normal ALDP. The ABCD1 gene is under the control of an internal MNDU3 promoter, which is a modified viral promoter and has been shown to control expression of the transgene in HSCs and their progeny in all lineages.
The transduced CD34+ cells are washed, formulated into a suspension, and then cryopreserved. SKYSONA is frozen in a patient-specific infusion bag(s) and is thawed prior to administration [see Dosage and Administration (2.1), How Supplied/Storage and Handling (16)]. The thawed product is colorless to white to red, including shades of white or pink, light yellow, and orange suspension of cells and may contain small proteinaceous particles. Due to the presence of cells, the solution may be clear to slightly cloudy and may contain visible cell aggregates.
The formulation contains 5% dimethyl sulfoxide (DMSO).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SKYSONA adds functional copies of the ABCD1 cDNA into patients' hematopoietic stem cells (HSCs) through transduction of autologous CD34+ cells with Lenti-D LVV. After SKYSONA infusion, transduced CD34+ HSCs engraft in the bone marrow and differentiate into various cell types, including monocytes (CD14+) capable of producing functional ALDP. Functional ALDP can then participate in the local degradation of very long chain fatty acids (VLCFAs), which is believed to slow or possibly prevent further inflammation and demyelination.
12.2 Pharmacodynamics
All patients who received SKYSONA with at least 1 month of follow-up produced ALDP in CD14+ cells (N=23, Study 1; N=31, Study 2), demonstrating early expression of the transgene. The %ALDP+ cell counts stabilized at 6 months after SKYSONA infusion. Patients had a Month 6 median (min, max) %ALDP+ CD14+ cells of 16% (2%, 71%) in Study 1 (N=23) and 26% (2%, 86%) in Study 2 (N=25).
In peripheral blood, the %ALDP+ CD14+ cells remained generally stable through Month 24 with a median (min, max) of 15% (6%, 45%) in Study 1 (N=23) and 28% (2%, 40%) in Study 2 (N=11). ALDP expression was quantifiable in 43% of the patients who had follow-up through Month 60 in Study 1 (N=7), indicating long-term expression of transgenic ALDP in the progeny of hematopoietic stem cells.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The safety and efficacy of SKYSONA were assessed in two 24-month, open-label, single-arm studies in patients with early, active CALD as defined by Loes score between 0.5 and 9 (inclusive) and gadolinium enhancement (GdE+) on MRI, as well as a neurologic function score (NFS) of ≤ 1, indicating limited changes in neurologic function. The NFS was used to evaluate 15 domains of neurological function with a maximum score of 25. A total NFS=0 indicates absence of neurologic dysfunction or asymptomatic disease. The patients enrolled and treated with SKYSONA (Study 1, N=32; Study 2, N=35) all had elevated very long chain fatty acid (VLCFA) levels and confirmed mutations in the ABCD1 gene. Following completion of Study 1 and Study 2, patients enroll in a subsequent and ongoing long-term follow-up study. The efficacy of SKYSONA was compared to an external untreated natural history control. Data for the Natural History Population in the retrospective natural history study (Study 3) was collected from existing medical records for patients with CALD. The Natural History Population had early, active disease at diagnosis, though gadolinium status was defined by either having a GdE+ MRI during the study or unknown GdE status and clinical course that suggested active disease.
SKYSONA Studies
Study 1 is complete and Study 2 is ongoing at the time of product approval. In Study 1, patients were 47% White/Caucasian, 38% Hispanic, 3% Asian, 3% Black or African American, and 16% other races including mixed race. In Study 2, patients were 60% White/Caucasian, 14% Hispanic, 6% Black or African American, 6% other races including mixed race.
Mobilization and Apheresis:
- G-CSF 10 µg/kg (median) for a minimum of 4 days
- Plerixafor 0.24 mg/kg for up to 3 days – optional in Study 1 (administered to 34% of patients) and required in Study 2
For all patients, one cycle of mobilization and apheresis and one to two apheresis collection days were sufficient to obtain the requisite number of cells needed for manufacturing.
Pre-treatment Myeloablative Conditioning:
- Study 1: Busulfan dose median (min, max) 14 (11.2 to 16.8) mg/kg over 4 days
- Study 2: Busulfan dose median (min, max) 16.8 (12 to 21.2) mg/kg over 4 days
Pre-treatment Lymphodepletion:
- Study 1: Cyclophosphamide dose median (min, max) 199 (151 to 213) mg/kg over 4 days
- Study 2: Fludarabine dose 180 mg/m2 over 6 days for 11 patients; 160 mg/m2 over 4 days (actual dose range 122 to 196 mg/m2) for 24 patients; (fludarabine dose decreased due to viral infections in the initial cohort)
Patients received seizure, hepatic veno-occlusive disease, anti-fungal, and antibiotic prophylaxis in accordance with institutional guidelines.
SKYSONA Administration:
- All patients were administered SKYSONA as an intravenous infusion with a median (min, max) dose of 12 × 106 (5, 38.2) CD34+ cells/kg (N=67).
After SKYSONA Administration:
- G-CSF – optional in Study 1 (administered to 75% of patients) and required in Study 2 (beginning on Day 5)
- See Section 6 for engraftment information.
Comparison of SKYSONA with the Natural History of CALD
A post-hoc enrichment analysis in symptomatic patients compared time from onset of symptoms (NFS ≥ 1) to time to first Major Functional Disability (MFD) or death (i.e., MFD-free survival) in SKYSONA treated and Natural History patients. The MFDs are defined as: loss of communication, cortical blindness, requirement for tube feeding, total incontinence, wheelchair dependence, or complete loss of voluntary movement. To be included in the analysis, patients had to have symptoms at baseline (NFS=1) or be asymptomatic (NFS=0) at baseline and have developed symptoms (NFS ≥ 1) during the course of follow-up in the study. Additionally, they had to have at least 24 months of follow-up after initial NFS ≥ 1 or have had an event (MFD or death).
The 7 patients in the Natural History Population were a median (min, max) 9 (5, 15) years old at time of CALD diagnosis, and 10 (5, 17) years at time of first NFS ≥ 1. The median Loes score at diagnosis was 5 (2, 9). Four (57%) had a baseline brain MRI pattern of disease inclusive of parieto-occipital involvement, 2 (29%) had frontal disease (without parieto-occipital involvement) and 1 (14%) had isolated pyramidal tract disease. One (14%) had a baseline NFS=1 at diagnosis, and the remainder were asymptomatic (NFS=0) at diagnosis.
The symptomatic SKYSONA subpopulation (N=11) had baseline median (min, max) age at treatment of 6 (4, 10) years, age at first NFS ≥ 1 of 7 (4, 10) years, and a baseline Loes score of 2.5 (1, 9). Ten (91%) patients had a parieto-occipital pattern of disease on brain MRI and 1 (9%) had isolated pyramidal tract disease. At baseline, 2 (18%) patients had an NFS=1 and the remainder were asymptomatic (NFS=0) prior to treatment.
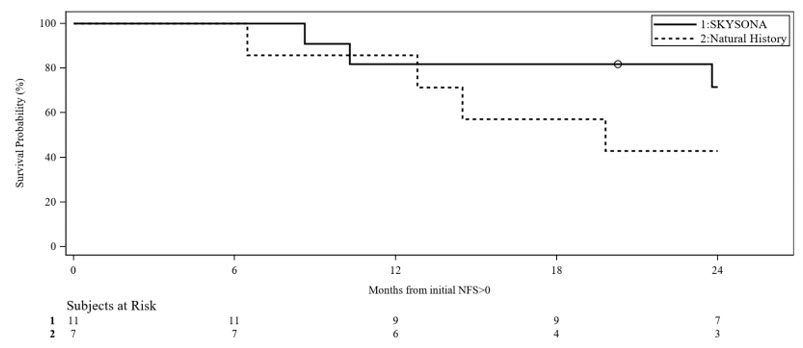
Slower progression to MFD or death from time of symptom onset (first NFS ≥ 1) was seen for early, active CALD patients treated with SKYSONA compared to a similar natural history of disease (Figure 1). Kaplan-Meier (KM) estimated MFD-free survival at Month 24 from time of first NFS ≥ 1 were 72% (95% CI: 35%, 90%) for the symptomatic SKYSONA subpopulation and 43% (95% CI: 10%, 73%) for the Natural History Population. There were insufficient data beyond 24 months for the symptomatic SKYSONA subpopulation to assess long-term MFD-free survival as compared to the natural history of disease. There was insufficient duration of follow-up to assess efficacy in SKYSONA treated patients who remained asymptomatic.
Figure 1 Kaplan-Meier Curve of MFD-free Survival in Symptomatic Patients of SKYSONA and Natural History Populations
Isolated Pyramidal Tract Disease
Two untreated patients in Study 3 had early CALD with isolated pyramidal tract disease on brain MRI. Both remained asymptomatic for approximately 10 years following CALD diagnosis with first symptoms documented at 19 and 20 years of age. Ten patients with early, active pyramidal tract disease were treated with SKYSONA in Studies 1 and 2, and have only been followed a maximum of 77 months following treatment and to a maximum age at last follow-up of 15 years. Two (20%) SKYSONA-treated patients were diagnosed with myelodysplastic syndrome (MDS) and received allo-HSCT as treatment of the hematologic malignancy. One (10%) patient developed symptoms and worsening lesions on brain MRI approximately 6 months following treatment with SKYSONA and was withdrawn from the study to receive allo-HSCT at the investigator's discretion. He subsequently died of transplant-related causes.
Comparison of SKYSONA with Allogeneic Hematopoietic Stem Cell Transplant (allo-HSCT)
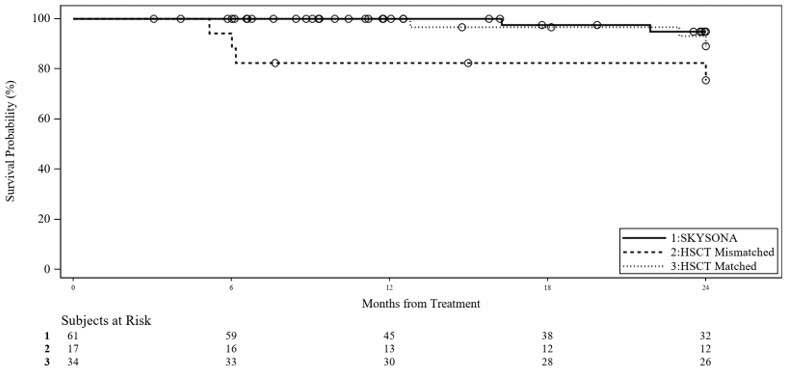
There were insufficient data to compare relative efficacy of SKYSONA to the standard of care, allogeneic hematopoietic stem cell transplant (allo-HSCT) in the treatment of CALD. However, while it does not inform the efficacy analysis, comparison of SKYSONA with an external allo-HSCT control (pooled from Study 3 and from a mixed prospective and retrospective allo-HSCT data collection study, Study 4) was performed for overall survival (OS) due to concerns about treatment-related toxicities. OS was analyzed as time-to-event Kaplan-Meier estimates comparing SKYSONA (entire efficacy population, N=61) to early, active allo-HSCT subpopulations by donor type: human leukocyte antigen (HLA)-Matched allo-HSCT Subpopulation (N=34) and HLA-Mismatched allo-HSCT Subpopulation (N=17) (Figure 2). There were insufficient long-term data to compare OS beyond Month 24. However, a distinct difference in OS in the first 9 months following treatment was seen for the subpopulation who received allo-HSCT from an HLA-mismatched donor as compared to SKYSONA and allo-HSCT from an HLA-matched donor. While this analysis does not provide evidence of efficacy of SKYSONA, it does demonstrate a survival advantage of SKYSONA as compared to allo-HSCT from an HLA-mismatched donor, with early mortality in the HLA-mismatched allo-HSCT Subpopulation largely attributed to allo-HSCT-related toxicities.
No patient experienced acute (≥ Grade II) or chronic graft versus host disease (GVHD) after SKYSONA treatment.
Figure 2 Kaplan-Meier Curve of Overall Survival Between SKYSONA and Allo-HSCT Treated Populations
-
16 HOW SUPPLIED/STORAGE AND HANDLING
SKYSONA is supplied in one or two infusion bags containing a frozen suspension of genetically modified autologous cells enriched for CD34+ cells. Each bag contains approximately 20 mL. Each SKYSONA infusion bag is individually packed within an overwrap in a metal cassette. SKYSONA is shipped from the manufacturing facility to the infusion center in a cryoshipper, which may contain multiple metal cassettes intended for treatment of a single patient. A Lot Information Sheet is affixed inside the shipper.
- 20 mL infusion bag, overwrap, and metal cassette (NDC 73554-2111-1)
Match the identity of the patient with the patient identifiers on the metal cassette(s), infusion bag(s), and Lot Information Sheet upon receipt.
- Store SKYSONA frozen in the vapor phase of liquid nitrogen at less than or equal to -140°C (-220°F) until ready for thaw and administration.
- Thaw SKYSONA prior to infusion [see Dosage and Administration (2)].
- Do not re-freeze after thawing.
- Do not irradiate SKYSONA as this could lead to inactivation.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and their caregiver(s) to read the FDA-approved patient labeling (Medication Guide).
Discuss the following with the patient and their caregiver(s):
Hematologic Malignancy
Inform the patient and/or caregiver that insertional oncogenesis has been observed and resulted in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in children following treatment with SKYSONA. Inform the patient that these are life-threatening diseases and children have died from complications of treatment for malignancy. Treatment for hematologic malignancies can include chemotherapy and bone marrow transplant.
Patients treated with SKYSONA will need lifelong monitoring for hematologic malignancies via blood tests. Advise patients that they may require more frequent blood tests and invasive bone marrow biopsies if routine blood test results are concerning for the development of malignancy [see Warnings and Precautions (5.1)]. Advise the patient and/or caregiver to seek attention for any signs or symptoms of malignancy, such as fatigue or easy bleeding or bruising, and advise the patient to contact bluebird bio at 1-833-999-6378 if they are diagnosed with a hematologic malignancy.
Efficacy Failure
Inform the patient and/or caregiver that there has been unexplained efficacy failure after treatment with SKYSONA [see Use in Specific Populations (8.6)].
Need for Allogeneic Hematopoietic Stem Cell Transplant
Inform the patient that some patients have required an allogeneic hematopoietic stem cell transplant after treatment with SKYSONA, in order to treat efficacy failure or hematologic malignancy. Advise the patients that they will be monitored for these complications [see Warnings and Precautions (5.1)].
Serious Infections
Inform the patient that severe infections, including life-threatening infections, have occurred following SKYSONA treatment and may require prolonged hospitalization [see Warnings and Precautions (5.2)].
Prolonged Cytopenias
Inform the patient and/or caregiver that prolonged cytopenias have been observed following SKYSONA treatment and that they may require frequent blood draws until blood counts have returned to safe levels. Advise the patient and/or caregiver to seek attention for any signs or symptoms of thrombocytopenia, neutropenia, or anemia, such as easy bleeding, serious infections, or fatigue [see Warnings and Precautions (5.3)].
Delayed Platelet Engraftment
Inform the patient that a risk of bleeding and a likely need for platelet transfusion exists after myeloablative conditioning and during the weeks to months before platelet engraftment occurs [see Warnings and Precautions (5.4)].
Risk of Neutrophil Engraftment Failure
Inform the patient and/or caregiver of the potential risk of neutrophil engraftment failure and the need for rescue treatment with their back-up collection of CD34+ cells, if engraftment failure occurs [see Warnings and Precautions (5.5)].
Laboratory Test Interference
Advise patients that they may test positive for HIV if tested using a PCR test after being treated with SKYSONA [see Warnings and Precautions (5.8)]. Advise the patient and/or caregiver that they should notify any healthcare provider about this possibility prior to being tested for HIV.
Manufacturing Failure
Ensure that the patient and/or caregiver understands the risk of manufacturing failure. In case of manufacturing failure or the need for additional cells, additional cell collection and manufacturing of SKYSONA may be needed [see Dosage and Administration (2.2)].
Advise patients that they will be screened for HBV, HCV, HIV, and HTLV before collection of cells [see Dosage and Administration (2.2)].
Inform patients that they should not donate blood, organs, tissues, or cells at any time in the future [see Dosage and Administration (2.3)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
SKYSONA® (pronounced sky-SO-nuh)
(elivaldogene autotemcel)This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: April 2024What is the most important information I or my caregiver should know about SKYSONA?
SKYSONA may cause cancer of the blood and bone marrow, which can be life-threatening and lead to death. Blood cancer has resulted from treatment with SKYSONA because cancer-causing genes have been turned on by the gene therapy. Patients have developed cancer as early as one year after SKYSONA administration, prior to SKYSONA having time to potentially help their CALD. Blood cancer can also take years to develop, and has been diagnosed as late as 7.5 years later. Because SKYSONA was approved while patients were still being evaluated for the risk of cancer, the percent of patients who will develop cancer and the maximum timeframe when cancer caused by SKYSONA could develop is unknown. Blood cancer is usually treated with chemotherapy and may require stem cell transplant. Stem cell transplant using cells from a donor have been used to treat children diagnosed with blood cancer that was caused by SKYSONA.
Blood cancers, including those following SKYSONA treatment, will lead to death if not treated. Treatment of blood cancer has led to death due to complications. Because of the risk of cancer caused by SKYSONA, your doctor may recommend that you are evaluated by a hematologist to determine if you have underlying risk factors that could further increase your risk for cancer and change whether SKYSONA is appropriate for you.
Before you are treated with SKYSONA, you should have a detailed discussion with your doctor about the risks and benefits of SKYSONA and alternative treatment options. Alternative treatment may include an allogeneic hematopoietic stem cell transplant. Discuss with your physician the possibility of a stem cell transplant using cells from a suitable and willing matched sibling donor if one is available.
Because of the risk of cancer, it is important for you to be monitored lifelong. At a minimum we recommend blood tests every 3 months for 15 years. Blood tests will look at your blood cell counts and the locations in your blood cells where the gene therapy is inserted. If your blood counts are too low or too high, or if you have lots of cells with the same gene therapy insertion sites, additional testing may be recommended. Additional testing might include more frequent blood tests to watch you more closely for changes in your blood. Additional testing could also include a bone marrow evaluation, which can tell your doctor more than blood tests about the health of your bone marrow and if there is cancer forming.
If blood cancer develops quickly or if you have not been having the recommended blood or bone marrow tests, you might experience symptoms of cancer before it is diagnosed. You or your caregiver should call your healthcare provider right away for any of these signs or symptoms:- Abnormal bruising or bleeding (including nosebleed)
- Blood in urine, stool, or vomit
- Coughing up blood
- Severe headache
- Unusual stomach or back pain
- Fever (100.4°F/38°C or higher)
- Swollen glands
- Abnormal tiredness
SKYSONA may cause life-threatening allergic reactions as it contains DMSO, a commonly used preserving agent. Please inform your healthcare provider if you have been told that your child has a DMSO allergy or has experienced a reaction after receiving a DMSO-containing product.
It is important that you or your caregiver tell your healthcare providers that you have received SKYSONA.What is SKYSONA?
SKYSONA is a one-time gene therapy to treat boys with early, active cerebral adrenoleukodystrophy (CALD). CALD is a genetic disease caused by mutations in the ABCD1 gene that lead to the buildup of very long chain fatty acids (VLCFAs) in the brain. These VLCFAs may destroy the protective covering around nerve cells and cause damage to the brain. Once this occurs, this damage can be seen on magnetic resonance imaging (MRI) of the brain, which is when the cerebral form of adrenoleukodystrophy (CALD) is diagnosed. SKYSONA may be recommended if this damage is determined to be early (based on a lesser degree of the damage on MRI) and if cerebral disease is active (based on presence of contrast enhancement on MRI that indicates this damage is ongoing). SKYSONA is made specifically for each patient, using the patient's own blood stem cells and adds functional copies of the ABCD1 gene to your cells. This may help your body to break down the VLCFAs to slow the progression of damage to the brain and slow the decline in neurologic function.How will I get SKYSONA?
Before treatment: Your healthcare providers will give you other medicines, including chemotherapy medicine, as part of your treatment before you are given SKYSONA. It's important that you or your caregiver talk to your healthcare providers about the risks and benefits of all medicines involved in your treatment.
After receiving the chemotherapy, it may not be possible for you to father a child. You or your caregiver should consider discussing options for fertility preservation with your doctor before treatment.
STEP 1: SKYSONA is made specifically for you from your own blood stem cells. Your healthcare provider will collect your blood stem cells through a process called mobilization and apheresis (A-feh-REE-sis). This process takes approximately one week.
'Back-up' stem cells (or 'rescue cells') are also collected and stored at the hospital. This is a precaution in case there is a problem during the treatment process. If this happens, your back-up stem cells will be given back to you. If you receive back-up cells, you will have no benefit from SKYSONA.
STEP 2: Your blood stem cells will be sent to a manufacturing site where they are used to make SKYSONA. It takes approximately 51 – 65 days from the time your cells are collected to make and test SKYSONA before it is shipped to your healthcare providers, but the time may vary.
STEP 3: Before you receive SKYSONA, your healthcare providers will give you chemotherapy for a few days to make room in the bone marrow. You will be admitted to the hospital for this step and remain in the hospital until after SKYSONA infusion.
STEP 4: SKYSONA is given by an intravenous infusion (into your vein). You may receive one or two bags of SKYSONA. Each bag is infused in less than 60 minutes.
After SKYSONA infusion, you may stay in the hospital for up to approximately 2 months so that your healthcare team can closely monitor your recovery. Your healthcare providers will determine when you can go home.What should I avoid after receiving SKYSONA? - Do not donate blood, organs, tissues or cells.
What are the possible or reasonably likely side effects of SKYSONA?
There is a risk of blood cancer following treatment with SKYSONA which will require lifelong monitoring. You will be monitored at least every 3 months for a minimum of 15 years for this.
Possible or reasonably likely side effects when treated with SKYSONA are:- While receiving chemotherapy to prepare your body for SKYSONA:
- Nausea, vomiting, decreased appetite, constipation, abdominal pain
- Headache
- Rash
- On the day of treatment with SKYSONA
- Life-threatening allergic reaction
- Nausea, vomiting
- Following treatment
- Blood cancer. Refer to "What is the most important information I or my caregiver should know about SKYSONA?"
- Low blood counts leading to a risk of bleeding and/or infection. Until your blood counts (platelets, white blood cells, red blood cells) return to safe levels, you may be treated with blood and platelet transfusions and other medicines that prevent bleeding and infection by increasing your blood counts. Most patients' blood counts return to safe levels in about one month after treatment with SKYSONA. Some patients' blood counts may not recover for > 1 year. Tell your healthcare provider right away if you get a fever, are feeling tired, or have easy bleeding or bruising.
- Life-threatening infections. Patients treated with SKYSONA may experience serious or life-threatening infections, including infections of the bloodstream by bacteria or viruses. Infections often occur in the first 1 or 2 months after treatment with SKYSONA, but can occur > 1 year later. Tell your healthcare provider right away if you develop fever, chills, or any signs or symptoms of an infection.
- Inflamed and painful mouth (this typically occurs during the first 2 months after SKYSONA treatment)
- Nausea, vomiting, decreased appetite, constipation, abdominal pain, diarrhea
- Headache
- New onset seizures
General information about the safe and effective use of SKYSONA.
Because of the risk of blood cancer, it is important for you to be monitored lifelong. At a minimum we recommend blood tests every 3 months for 15 years.
SKYSONA treatment failures have been reported and progression of CALD may occur. After treatment with SKYSONA you will need to continue to follow-up with your doctor who will monitor you for any clinical and radiographic worsening of CALD.
Treatment with SKYSONA may cause a false-positive human immunodeficiency virus (HIV) test result by some commercial tests. If you need to have an HIV test, talk with your healthcare provider about the appropriate test to use.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Talk to your healthcare provider about any concerns. You can ask your healthcare provider for information about SKYSONA that is written for healthcare professionals.
For more information, go to SKYSONA.com or call 1-833-666-2583 for bluebird Patient Services (my bluebird support).
Manufactured for and distributed by: bluebird bio, Inc., Somerville, Massachusetts 02145 -
PRINCIPAL DISPLAY PANEL - 20 mL Bag Patient Identifier Label
elivaldogene autotemcel
skysona™Suspension for IV infusion
20 mL containing 4 to 30 x 106 cells/mL
(3.6 to 30 x 106 CD34+ cells/mL)Confirm Patient Identifiers
Last Name: $LastName$
First Name: $FirstName$
Date of Birth: $DOB$
bbb Patient ID: $bbb_PatientID$
COI ID: $bbb_COI_ID$
LOT: $LOT$
EXP: $Expiry$
Bag X of X
DIN: $DIN1_DIN2$
U.S. Lic. # 2160
Label P/N: XXXXXXX
bluebirdbio®
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Label
elivaldogene autotemcel
skysona™Suspension for IV infusion
20 mL containing 4 to 30 x 106 cells/mL
(3.6 to 30 x 106 CD34+ cells/mL)For autologous use only. For intravenous use only. Rx only.
Contains genetically modified autologous hematopoietic stem cells
suspended in cryopreservation solution containing 5% DMSO.Not evaluated for infectious substances.
Do not irradiate. Do not use an in-line blood filter or infusion pump.
See full prescribing information for dosage and administration.
See Lot Information Sheet for number of infusion bags and
CD34+ cells per kg for this patient. Dispense with Medication Guide.P/N: XXXXXXX
Label P/N: XXXXXXXManufactured for: bluebird bio, Inc.
Somerville, MA 02145
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Cassette Label
elivaldogene autotemcel
skysona™Suspension for IV infusion
20 mL containing 4 to 30 x 106 cells/mL
(3.6 to 30 x 106 CD34+ cells/mL)For autologous use only. For intravenous use only. Rx only.
Contains autologous hematopoietic stem cells transduced with Lenti-D
lentiviral vector suspended in cryopreservation solution containing 5% DMSO.Keep infusion bag(s) in the metal cassette(s). Store in the vapor phase of
liquid nitrogen at ≤ -140°C until ready for thaw and administration. Once
thawed do not re-freeze.See full prescribing information for dosage and administration.
Do not irradiate. Do not use an in-line blood filter or infusion pump.
This medicine contains genetically modified cells.
Not evaluated for infectious substances. No preservatives.
See Lot Information Sheet for number of infusion bags and CD34+ cells
per kg for this patient. Dispense with Medication Guide.Confirm Patient Identifiers
Last Name: $LastName$
First Name: $FirstName$
Date of Birth: $DOB$
bbb Patient ID: $bbb_PatientID$
COI ID: $bbb_COI_ID$
DIN: $DIN1_DIN2$
LOT: $LOT$
EXP: $Expiry$
Bag X of X
U.S. Lic. # 2160
Manufactured for: bluebird bio, Inc.
Somerville, MA 02145
1-833-999-6378
SKYSONA.comP/N: XXXXXXX
Label P/N: XXXXXXXbluebirdbio®

-
INGREDIENTS AND APPEARANCE
SKYSONA
elivaldogene autotemcel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:73554-2111 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength elivaldogene autotemcel (UNII: KUM75TD6SG) (elivaldogene autotemcel - UNII:KUM75TD6SG) elivaldogene autotemcel 30000000 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73554-2111-1 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125755 09/16/2022 Labeler - bluebird bio, Inc. (969116102) Establishment Name Address ID/FEI Business Operations Lonza Houston, Inc. 832903004 ANALYSIS(73554-2111) , LABEL(73554-2111) , MANUFACTURE(73554-2111) , PACK(73554-2111) , API MANUFACTURE(73554-2111)