Label: EXKIVITY- mobocertinib capsule
- NDC Code(s): 63020-040-12, 63020-040-90
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EXKIVITY safely and effectively. See full prescribing information for EXKIVITY.
EXKIVITY® (mobocertinib) capsules, for oral use
Initial U.S. Approval: 2021WARNING: QTc PROLONGATION AND TORSADES DE POINTES
See full prescribing information for complete boxed warning.
- EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal, and requires monitoring of QTc and electrolytes at baseline and periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation (5.1).
- Avoid use of concomitant drugs which are known to prolong the QTc interval and use of strong or moderate CYP3A inhibitors with EXKIVITY, which may further prolong the QTc (5.1, 7.1, 7.3).
- Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of QTc prolongation (2.4).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
EXKIVITY is a kinase inhibitor indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1, 2.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 40 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis. Immediately withhold EXKIVITY in patients with suspected ILD/pneumonitis and permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed. (2.4, 5.2)
- Cardiac Toxicity: Monitor cardiac function, including left ventricular ejection fraction, at baseline and during treatment. Withhold, resume at reduced dose or permanently discontinue based on severity. (2.4, 5.3)
- Diarrhea: Diarrhea may lead to dehydration or electrolyte imbalance, with or without renal impairment. Monitor electrolytes and advise patients to start an antidiarrheal agent at first episode of diarrhea and to increase fluid and electrolyte intake. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity. (2.4, 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective non-hormonal contraception. (5.5, 8.1, 8.3)
ADVERSE REACTIONS
The most common (>20%) adverse reactions are diarrhea, rash, stomatitis, vomiting, decreased appetite, paronychia, nausea, musculoskeletal pain, dry skin, fatigue, cough, pruritus, and decreased weight. The most common (≥2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes, increased amylase, increased lipase, decreased potassium, decreased red blood cells, increased creatinine, decreased magnesium and increased alanine aminotransferase. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-844-217-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: QTc PROLONGATION AND TORSADES DE POINTES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluation and Testing Before Initiating EXKIVITY
2.2 Patient Selection
2.3 Recommended Dosage
2.4 Dosage Modifications for Adverse Reactions
2.5 Dosage Modifications for Moderate CYP3A Inhibitors
2.6 Dosage Modifications for Patients with Severe Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 QTc Prolongation and Torsades de Pointes
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
5.3 Cardiac Toxicity
5.4 Diarrhea
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on EXKIVITY
7.2 Effect of EXKIVITY on Other Drugs
7.3 Drugs that Prolong the QTc Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: QTc PROLONGATION AND TORSADES DE POINTES
- EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal, and requires monitoring of QTc and electrolytes at baseline and periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation [see Warnings and Precautions (5.1)].
- Avoid use of concomitant drugs which are known to prolong the QTc interval and use of strong or moderate CYP3A inhibitors with EXKIVITY, which may further prolong the QTc [see Warnings and Precautions (5.1), Drug Interactions (7.1, 7.3)].
- Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of QTc prolongation [see Dosage and Administration (2.4)].
-
1 INDICATIONS AND USAGE
EXKIVITY is indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)], whose disease has progressed on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluation and Testing Before Initiating EXKIVITY
Before initiating EXKIVITY, evaluate QTc and electrolytes and correct abnormalities in sodium, potassium, calcium, and magnesium [see Warnings and Precautions (5.1)].
2.2 Patient Selection
Select patients with locally advanced or metastatic NSCLC for treatment with EXKIVITY based on the presence of EGFR exon 20 insertion mutations [see Clinical Studies (14)]. Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics.
2.3 Recommended Dosage
The recommended dosage of EXKIVITY is 160 mg orally once daily until disease progression or unacceptable toxicity.
Take EXKIVITY with or without food [see Clinical Pharmacology 12.3], at the same time each day. Swallow EXKIVITY capsules whole. Do not open, chew or dissolve the contents of the capsules.
If a dose is missed by more than 6 hours, skip the dose and take the next dose the following day at its regularly scheduled time.
If a dose is vomited, do not take an additional dose. Take the next dose as prescribed the following day.
2.4 Dosage Modifications for Adverse Reactions
EXKIVITY dose reduction levels for adverse reactions are summarized in Table 1.
Table 1: Recommended EXKIVITY Dose Reductions Dose Reductions Dose Level First dose reduction 120 mg once daily Second dose reduction 80 mg once daily Recommended dosage modifications of EXKIVITY for adverse reactions are provided in Table 2.
Table 2: Recommended Dosage Modifications for EXKIVITY Adverse Reactions Adverse Reaction Severity* EXKIVITY Dosage Modification ULN = upper limit of normal - *
- Graded per Common Terminology Criteria for Adverse Events Version 5.0
QTc Interval Prolongation and Torsades de Pointes
[see Warnings and Precautions (5.1)]Grade 2
(QTc interval 481-500 msec)First Occurrence - Withhold EXKIVITY until ≤ Grade 1 or baseline.
- Upon recovery, resume EXKIVITY at the same dose.
- Withhold EXKIVITY until ≤ Grade 1 or baseline.
- Upon recovery, resume EXKIVITY at the next lower dose or permanently discontinue EXKIVITY.
Grade 3
(QTc interval ≥501 msec or QTc interval increase of >60 msec from baseline)First Occurrence - Withhold EXKIVITY until ≤ Grade 1 or baseline.
- Upon recovery, resume EXKIVITY at the next lower dose or permanently discontinue EXKIVITY.
- Permanently discontinue EXKIVITY.
Grade 4
(Torsades de Pointes; polymorphic ventricular tachycardia; signs/symptoms of serious arrhythmia)- Permanently discontinue EXKIVITY.
Interstitial Lung Disease (ILD)/pneumonitis
[see Warnings and Precautions (5.2)]Any grade - Withhold EXKVITY if ILD/pneumonitis is suspected.
- Permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed.
Decreased Ejection Fraction or Heart Failure
[see Warnings and Precautions (5.3)]Grade 2 decreased ejection fraction - Withhold EXKIVITY until ≤ Grade 1 or baseline.
- If recovered to baseline within 2 weeks, resume EXKIVITY at the same dose or the next lower dose.
- If not recovered to baseline within 2 weeks, permanently discontinue EXKIVITY.
≥ Grade 2 heart failure or Grade 3 or 4 decreased ejection fraction - Permanently discontinue EXKIVITY.
Diarrhea
[see Warnings and Precautions (5.4)]Intolerable or recurrent Grade 2 or Grade 3 - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the same dose or the next lower dose.
Grade 4 First Occurrence - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the next lower dose.
- Permanently discontinue EXKIVITY.
Increased Amylase or Lipase
[see Adverse Reactions (6.1)]Grade 3 without signs or symptoms - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the same dose or next lower dose.
- If not recovered to ≤ Grade 1 within 2 weeks, permanently discontinue EXKIVITY.
Grade 3 with signs or symptoms - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the next lower dose.
- If not recovered to ≤ Grade 1 within 2 weeks, permanently discontinue EXKIVITY.
Grade 4 First Occurrence - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the next lower dose if recovery occurs within 2 weeks.
- Permanently discontinue EXKIVITY if recovery does not occur within 2 weeks.
- Permanently discontinue EXKIVITY.
Other Adverse Reactions
[see Adverse Reactions (6.1)]Intolerable or recurrent Grade 2 or Grade 3 - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the same dose or the next lower dose.
Grade 4 First Occurrence - Withhold EXKIVITY until ≤ Grade 1.
- Resume EXKIVITY at the next lower dose if recovery occurs within 2 weeks.
- Permanently discontinue EXKIVITY if recovery does not occur within 2 weeks.
- Permanently discontinue EXKIVITY.
2.5 Dosage Modifications for Moderate CYP3A Inhibitors
Avoid concomitant use of moderate CYP3A inhibitors with EXKIVITY. If concomitant use of a moderate CYP3A inhibitor cannot be avoided, reduce the EXKIVITY dose by approximately 50% (i.e., from 160 to 80 mg, 120 to 40 mg, or 80 to 40 mg) and monitor the QTc interval more frequently. After the moderate CYP3A inhibitor has been discontinued for 3 to 5 elimination half-lives, resume EXKIVITY at the dose taken prior to initiating the moderate CYP3A inhibitor [see Drug Interactions (7.1)].
2.6 Dosage Modifications for Patients with Severe Renal Impairment
Reduce the EXKIVITY dose by approximately 50% (i.e., from 160 to 80 mg, 120 to 40 mg, or 80 to 40 mg) and monitor the QTc interval more frequently for patients with severe renal impairment [see Use in Specific Populations (8.6)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 QTc Prolongation and Torsades de Pointes
EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal. In the 250 patient subset of the pooled EXKIVITY safety population who had scheduled and unscheduled electrocardiograms (ECGs) [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)], 1.2% of patients had a QTc interval >500 msec and 11% of patients had a change-from-baseline QTc interval >60 msec. Grade 4 Torsades de Pointes occurred in 1 patient (0.4%). Clinical trials of EXKIVITY did not enroll patients with baseline QTc greater than 470 msec.
Assess QTc and electrolytes at baseline and correct abnormalities in sodium, potassium, calcium, and magnesium prior to initiating EXKIVITY. Monitor QTc and electrolytes periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation, such as patients with congenital long QT syndrome, heart disease, severe renal impairment, or electrolyte abnormalities. Avoid use of concomitant drugs which are known to prolong the QTc interval. Avoid concomitant use of strong or moderate CYP3A inhibitors with EXKIVITY [see Drug Interactions (7.1)], which may further prolong the QTc [see Drug Interactions (7.3)].
Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of the QTc prolongation [see Dosage and Administration (2.4)].
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
EXKIVITY can cause ILD/pneumonitis, which can be fatal. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], ILD/pneumonitis occurred in 4.3% of patients including 0.8% Grade 3 events and 1.2% fatal events.
Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis. Immediately withhold EXKIVITY in patients with suspected ILD/pneumonitis and permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed [see Dosage and Administration (2.4)].
5.3 Cardiac Toxicity
EXKIVITY can cause cardiac toxicity (including decreased ejection fraction, cardiomyopathy, and congestive heart failure) resulting in heart failure which can be fatal. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], heart failure occurred in 2.7% of patients including 1.2% Grade 3 reactions, 0.4% Grade 4 reactions, and one (0.4%) fatal case of heart failure.
EXKIVITY can cause QTc prolongation resulting in Torsades de Pointes [see Warnings and Precautions (5.1)]. Atrial fibrillation (1.6%), ventricular tachycardia (0.4%), first degree atrioventricular block (0.4%), second degree atrioventricular block (0.4%), left bundle branch block (0.4%), supraventricular extrasystoles (0.4%) and ventricular extrasystoles (0.4%) also occurred in patients receiving EXKIVITY.
Monitor cardiac function, including assessment of left ventricular ejection fraction at baseline and during treatment. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity [see Dosage and Administration (2.4)].
5.4 Diarrhea
EXKIVITY can cause diarrhea, which can be severe. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], diarrhea occurred in 93% of patients, including 20% Grade 3 and 0.4% Grade 4. The median time to first onset of diarrhea was 5 days but diarrhea has occurred within 24 hours after administration of EXKIVITY. In the 48% of patients whose diarrhea resolved, the median time to resolution was 3 days. Diarrhea may lead to dehydration or electrolyte imbalance, with or without renal impairment. Treat diarrhea promptly.
Advise patients to start an antidiarrheal agent (e.g., loperamide) at first sign of diarrhea or increased bowel movement frequency and to increase fluid and electrolyte intake.
Monitor electrolytes and withhold, reduce the dose or permanently discontinue EXKIVITY based on the severity [see Dosage and Administration (2.4)].
5.5 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, EXKIVITY can cause fetal harm when administered to a pregnant woman. Oral administration of mobocertinib to pregnant rats during the period of organogenesis resulted in embryolethality at maternal exposures approximately 1.7 times the human exposure based on area under the curve (AUC) at the 160 mg once daily clinical dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective non-hormonal contraception during treatment with EXKIVITY [see Drug Interactions (7.2)] and for 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with EXKIVITY and for 1 week after the last dose of EXKIVITY [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QTc Prolongation and Torsades de Pointes [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.2)]
- Cardiac Toxicity [see Warnings and Precautions (5.3)]
- Diarrhea [see Warnings and Precautions (5.4)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in WARNINGS AND PRECAUTIONS reflects exposure to EXKIVITY as a single agent at a dose of 160 mg orally once daily in 256 patients, including 114 patients with EGFR exon 20 insertion mutation-positive locally advanced or metastatic NSCLC from Study AP32788-15-101, and patients with other solid tumors. Forty-eight percent (48%) were exposed for 6 months or longer and 12% were exposed for greater than one year. The most common (>20%) adverse reactions were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain. The most common (≥2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes, increased amylase, increased lipase, decreased potassium, decreased hemoglobin, increased creatinine, and decreased magnesium.
EGFR Exon 20 Insertion Mutation-Positive Locally Advanced or Metastatic NSCLC Previously Treated with Platinum-Based Chemotherapy
The safety of EXKIVITY was evaluated in a subset of patients in Study AP32788-15-101 with EGFR exon 20 insertion mutation-positive locally advanced or metastatic NSCLC who received prior platinum-based chemotherapy [see Clinical Studies (14)]. Patients with a history of interstitial lung disease, drug-related pneumonitis, radiation pneumonitis that required steroid treatment; significant, uncontrolled, active cardiovascular disease; or prolonged QTc interval were excluded from enrollment in this trial. A total of 114 patients received EXKIVITY 160 mg once daily until disease progression or unacceptable toxicity; 60% were exposed for 6 months or longer and 14% were exposed for greater than 1 year.
Serious adverse reactions occurred in 46% of patients who received EXKIVITY. Serious adverse reactions in ≥2% of patients included diarrhea, dyspnea, vomiting, pyrexia, acute kidney injury, nausea, pleural effusion, and cardiac failure. Fatal adverse reactions occurred in 1.8% of patients who received EXKIVITY, including cardiac failure (0.9%), and pneumonitis (0.9%).
Permanent discontinuation occurred in 17% of patients who received EXKIVITY. Adverse reactions requiring permanent discontinuation of EXKIVITY in at least ≥2% of patients were diarrhea and nausea.
Dosage interruptions of EXKIVITY due to an adverse reaction occurred in 51% of patients. Adverse reactions which required dosage interruption in >5% of patients included diarrhea, nausea and vomiting.
Dose reductions of EXKIVITY due to an adverse reaction occurred in 25% of patients. The adverse reaction requiring dose reduction in >5% of patients was diarrhea.
Table 3 summarizes the adverse reactions in Study AP32788-15-101.
Table 3: Adverse Reactions (≥10%) in Patients with EGFR Exon 20 Insertion Mutation-Positive NSCLC Whose Disease Has Progressed on or after Platinum-Based Chemotherapy in Study AP32788-15-101 Adverse Reaction EXKIVITY
(N = 114)All Grades *
(%)Grade 3 or 4
(%)- *
- Graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 5)
- †
- Stomatitis includes angular cheilitis, aphthous ulcer, cheilitis, mouth ulceration, mucosal inflammation, odynophagia, and stomatitis.
- ‡
- Events of Grade 3 only (no Grade 4 occurred)
- §
- Abdominal pain includes abdominal discomfort, abdominal pain, abdominal pain upper, abdominal tenderness, and gastrointestinal pain.
- ¶
- Rash includes acne, dermatitis, dermatitis acneiform, rash, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular, and urticaria.
- #
- Paronychia includes nail bed tenderness, nail disorder, nail infection, onycholysis, and paronychia.
- Þ
- Musculoskeletal pain includes arthralgia, back pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, pain in extremity, and spinal pain.
- ß
- Fatigue includes asthenia, and fatigue.
- à
- Cough includes cough, productive cough, and upper-airway cough syndrome.
- è
- Upper respiratory tract infection includes nasopharyngitis, pharyngitis, respiratory tract infection, rhinitis, sinusitis, and upper respiratory tract infection.
- ð
- Dyspnea includes dyspnea, and dyspnea exertional.
- ø
- Ocular toxicity includes dry eye, eye pruritus, abnormal sensation in eye, eye discharge, blepharitis, trichiasis, conjunctival hemorrhage, vitreous floaters, blurred vision and corneal edema.
- ý
- QTc interval prolongation includes electrocardiogram QT prolonged, and ventricular arrhythmia.
- £
- Hypertension includes blood pressure increased, and hypertension.
Gastrointestinal Disorders Diarrhea 92 22 Stomatitis† 46 4.4‡ Vomiting 40 2.6‡ Decreased appetite 39 0.9‡ Nausea 37 4.4‡ Decreased weight 21 0 Abdominal pain§ 18 1.8‡ Gastroesophageal reflux disease 15 0 Dyspepsia 11 0 Skin and Subcutaneous Tissue Disorders Rash¶ 78 1.8‡ Paronychia# 39 0.9‡ Dry skin 32 0 Pruritus 24 0.9‡ Alopecia 19 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal painÞ 34 2.6‡ General Disorders and Administration Site Conditions Fatigueß 29 3.5‡ Respiratory, Thoracic and Mediastinal Disorders Coughà 24 0 Upper respiratory tract infectionè 16 0 Dyspneað 15 4.4 Rhinorrhea 13 0 Eye Disorders Ocular Toxicityø 11 0 Cardiac Disorders QTc interval prolongationý 10 3.5 Hypertension £ 10 4.4‡ Nervous System Disorders Headache 10 0 Clinically relevant adverse reactions in <10% of patients receiving EXKIVITY included edema (9%), acute kidney injury (8%), peripheral neuropathy (7%), palmar-plantar erythrodysesthesia (4.4%), pneumonitis (2.6%) and cardiac failure (2.6%).
Table 4 summarizes the laboratory abnormalities in Study AP32788-15-101.
Table 4: Select Laboratory Abnormalities (≥20%) Worsening from Baseline in Patients with EGFR Exon 20 Insertion Mutation-Positive NSCLC Whose Disease Has Progressed on or after Platinum-Based Chemotherapy in Study AP32788-15-101 Laboratory Abnormality EXKIVITY*
(N = 114)All Grades†
(%)Grade 3 or 4
(%)Hematology Decreased red blood cells 59 3.5 Decreased lymphocytes 52 15 Decreased platelets 26 0.9 Decreased leukocytes 25 0 Chemistry Increased creatinine 52 2.7 Increased amylase 40 13 Increased lipase 35 10 Decreased potassium 29 5 Increased alkaline phosphatase 25 1.8 Decreased albumin 23 1.8 Decreased magnesium 23 2.7 Increased alanine aminotransferase 22 2.7 Increased aspartate aminotransferase 21 1.8 Decreased sodium 20 0.9 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on EXKIVITY
Strong or Moderate CYP3A Inhibitors Clinical Impact - Coadministration of EXKIVITY with strong or moderate CYP3A inhibitors increased mobocertinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions, including QTc interval prolongation.
Prevention or Management - Avoid concomitant use of strong or moderate CYP3A inhibitors with EXKIVITY. If concomitant use of moderate CYP3A inhibitors cannot be avoided, reduce the EXKIVITY dose and monitor the QTc interval more frequently with ECGs [see Dosage and Administration (2.5), Warnings and Precautions (5.1)].
Strong or Moderate CYP3A Inducers Clinical Impact - Coadministration of EXKIVITY with strong or moderate CYP3A inducers decreased mobocertinib plasma concentrations [see Clinical Pharmacology (12.3)], which may reduce EXKIVITY anti-tumor activity.
Prevention or Management - Avoid concomitant use of strong or moderate CYP3A inducers with EXKIVITY.
7.2 Effect of EXKIVITY on Other Drugs
CYP3A Substrates Clinical Impact - Coadministration of EXKIVITY with CYP3A substrates may decrease plasma concentrations of CYP3A substrates [see Clinical Pharmacology (12.3)], which may reduce the efficacy of these substrates.
Prevention or Management - Avoid concomitant use of hormonal contraceptives with EXKIVITY [see Warnings and Precautions (5.5), Use in Specific Populations (8.3)].
- Avoid concomitant use of EXKIVITY with other CYP3A substrates where minimal concentration changes may lead to serious therapeutic failures. If concomitant use is unavoidable, increase the CYP3A substrate dosage in accordance with the approved product Prescribing Information.
7.3 Drugs that Prolong the QTc Interval
Drugs that Prolong the QTc Interval Clinical Impact - EXKIVITY can cause QTc interval prolongation [see Warnings and Precautions (5.1), Clinical Pharmacology (12.2)]. Coadministration of EXKIVITY with drugs known to prolong the QTc interval may increase the risk of QTc interval prolongation [see Warnings and Precautions (5.1), Clinical Pharmacology (12.2)].
Prevention or Management - Avoid concomitant use of other medications known to prolong the QTc interval with EXKIVITY. If concomitant use is unavoidable, monitor the QTc interval more frequently with ECGs [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], EXKIVITY can cause fetal harm when administered to a pregnant woman. There are no available data on EXKIVITY use in pregnant women. Oral administration of mobocertinib to pregnant rats during the period of organogenesis resulted in embryolethality (embryo-fetal death) and maternal toxicity at plasma exposures approximately 1.7 times the human exposure based on AUC at the 160 mg once daily clinical dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study, once daily oral administration of mobocertinib to pregnant rats during the period of organogenesis resulted in maternal toxicity (reduced body weight gain and food consumption) at 10 mg/kg (approximately 1.7 times the human exposure based on AUC at the 160 mg once daily clinical dose). Adverse effects on embryo-fetal development at this dose level included embryolethality due to post-implantation loss (embryo-fetal death) and effects on fetal growth (decreased fetal weights). There was no clear evidence of fetal malformations at the high dose level (10 mg/kg).
8.2 Lactation
Risk Summary
There are no data on the presence of mobocertinib or its metabolites in human milk or their effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with EXKIVITY and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
EXKIVITY can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating EXKIVITY.
Contraception
Females
Advise females of reproductive potential to use effective non-hormonal contraception during treatment with EXKIVITY and for 1 month after the last dose. EXKIVITY may render hormonal contraceptives ineffective [see Drug Interactions (7.2)].
Infertility
Based on animal studies, EXKIVITY may impair fertility in males and females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of EXKIVITY in pediatric patients have not been established.
8.5 Geriatric Use
Of the 114 patients [see Clinical Studies (14)] who received EXKIVITY in clinical studies, 37% were 65 years and over, and 7% were 75 years and over. No overall difference in effectiveness was observed between patients aged 65 and older and younger patients. Exploratory analysis suggests a higher incidence of Grade 3 and 4 adverse reactions (69% vs 47%) and serious adverse reactions (64% vs 35%) in patients 65 years and older as compared to those younger than 65 years.
8.6 Renal Impairment
Mobocertinib plasma concentrations are higher in patients with severe renal impairment [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions. Reduce the recommended dosage of EXKIVITY for patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2 by Modification of Diet in Renal Disease [MDRD] equation) [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)]. No dosage adjustment of EXKIVITY is recommended for patients with mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m2 by MDRD equation).
8.7 Hepatic Impairment
No dosage adjustment of EXKIVITY is recommended for patients with mild (total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] > ULN or total bilirubin >1 to 1.5 times ULN and any AST)-to-severe (total bilirubin >3 times ULN and any AST) hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
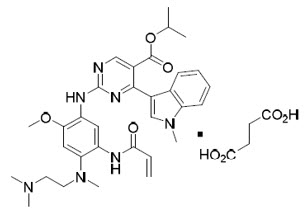
Mobocertinib is a kinase inhibitor. The chemical name for mobocertinib succinate is propan-2-yl 2-[5-(acryloylamino)-4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxyanilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate succinate. The molecular formula is C32H39N7O4 + C4H6O4 (succinate salt) which corresponds to a molecular weight of 703.8 g/mol. Mobocertinib has no chiral centers. The chemical structure of mobocertinib succinate is shown below:
Mobocertinib succinate has a solubility of 152 mg/mL in pH 1.0 and >17.6 mg/mL in pH 6.8 solutions at 37°C.
EXKIVITY capsule for oral administration contains 40 mg mobocertinib equivalent to 48.06 mg mobocertinib succinate, with no inactive ingredients. The capsule shells contain gelatin and titanium dioxide. The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mobocertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR) that irreversibly binds to and inhibits EGFR exon 20 insertion mutations at lower concentrations than wild type (WT) EGFR. Two pharmacologically-active metabolites (AP32960 and AP32914) with similar inhibitory profiles to mobocertinib have been identified in the plasma after oral administration of mobocertinib. In vitro, mobocertinib also inhibited the activity of other EGFR family members (HER2 and HER4) and one additional kinase (BLK) at clinically relevant concentrations (IC50 values <2 nM).
In cultured cell models, mobocertinib inhibited the proliferation of cells driven by different EGFR exon 20 insertion mutation variants at 1.5- to 10-fold lower concentrations than WT-EGFR signaling inhibition.
In animal tumor implantation models, mobocertinib exhibited anti-tumor activity against xenografts with the EGFR exon 20 insertions NPH or ASV.
12.2 Pharmacodynamics
Mobocertinib exposure-response relationships and the time course of pharmacodynamic response are unknown.
Cardiac Electrophysiology
The largest mean increase in QTc was 23.0 msec (UCI: 25.5 msec) following administration of EXKIVITY 160 mg once daily. The increase in QTc interval was concentration-dependent.
The largest mean increase in the PR interval was 12.4 msec (UCI: 15.0 msec). PR interval prolongation >220 msec occurred in 5% of patients taking EXKIVITY 160 mg once daily.
12.3 Pharmacokinetics
After single- and multiple-dose administration, combined molar Cmax and AUC0-24h of mobocertinib and its active metabolites, AP32960 and AP32914, was dose-proportional over the dose range of 5 to 180 mg once daily (0.03 to 1.1 times the approved recommended dosage). No clinically meaningful accumulation was observed after administration of EXKIVITY 160 mg once daily based on the AUC ratio of mobocertinib.
Absorption
The median (min, max) time to peak concentration (Tmax) of mobocertinib is 4 hours (1, 8 hours). The mean (%CV) absolute bioavailability is 37% (50%).
Effect of Food
No clinically meaningful differences in the combined molar AUC and Cmax of mobocertinib, AP32960, and AP32914 were observed following administration of a high-fat meal (approximately 900 to 1000 calories, with 150 calories from protein, 250 calories from carbohydrate and 500 to 600 calories from fat) or a low fat-meal (approximately 336 calories, with 37 calories from protein, 253 calories from carbohydrate, and 46 calories from fat) compared to administration after an overnight fast.
Distribution
Mobocertinib was bound to human plasma proteins in a concentration independent manner in vitro from 0.5 to 5.0 μM. The mean (standard deviation) bound fraction was 99.3% (0.11%) for mobocertinib, 99.5% (0.16%) for AP32960 and 98.6% (0.36%) for AP32914 in vitro.
The blood-to-plasma ratio was 0.76 for mobocertinib, 1.2 for AP32960 and 0.71 for AP32914.
The mean (%CV) apparent volume of distribution (Vss/F) of mobocertinib was 3,509 L (38%) at steady-state.
Elimination
The mean (%CV) plasma elimination half-life of mobocertinib was 18 hours (21%) at steady-state. The mean apparent oral clearance (CL/F) (%CV) of mobocertinib was 138 L/hr (47%) at steady-state.
The mean (%CV) plasma elimination half-life of AP32960 was 24 hours (20%) at steady-state. The mean apparent oral clearance (CL/F) (%CV) of AP32960 was 149 L/hr (36%) at steady-state.
The mean (%CV) plasma elimination half-life of AP32914 was 18 hours (21%) at steady-state. The mean apparent oral clearance (CL/F) (%CV) of AP32914 was 159 L/hr (52%) at steady-state.
Metabolism
Mobocertinib is primarily metabolized by CYP3A. The two active metabolites, AP32960 and AP32914, are equipotent to mobocertinib and account for 36% and 4% of the combined molar AUC, respectively.
Excretion
Following administration of a single 160 mg oral dose of radiolabeled mobocertinib, approximately 76% of the dose was recovered in feces (approximately 6% as unchanged mobocertinib) and approximately 4% was recovered in urine (approximately 1% as unchanged mobocertinib). The percentage of the administered dose recovered in feces and urine for AP32960 was approximately 12% and 1%, respectively. The metabolite AP32914 was below the detection limit in urine and feces.
Specific Populations
No clinically meaningful differences in the pharmacokinetics of mobocertinib were observed based on age (18 to 86 years), race (White, Black, Asian), sex, body weight (37.3 to 132 kg), mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m2 by MDRD equation), or mild (total bilirubin ≤ ULN and AST > ULN or total bilirubin >1 to 1.5 times ULN and any AST)-to-severe (total bilirubin >3 times ULN and any AST) hepatic impairment.
Patients with Renal Impairment
Following administration of a single 80 mg oral dose of EXKIVITY, the unbound combined molar AUC of mobocertinib and its active metabolites was increased by 112% in subjects with severe renal impairment (eGFR <30 mL/min/1.73 m2 by MDRD equation) as compared to subjects with normal renal function (eGFR ≥90 mL/min/1.73 m2 by MDRD equation).
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of CYP3A Inhibitors on Mobocertinib: Coadministration of EXKIVITY with multiple doses of itraconazole or ketoconazole (strong CYP3A inhibitors) is predicted to increase the steady-state combined molar AUC of mobocertinib and its active metabolites by 374 to 419%.
Coadministration of EXKIVITY with multiple doses of a moderate CYP3A inhibitor is predicted to increase the steady-state combined molar AUC of mobocertinib and its active metabolites by approximately 100-200%.
Effect of CYP3A Inducers on Mobocertinib: Coadministration of EXKIVITY with multiple doses of rifampin (a strong CYP3A inducer) is predicted to decrease the steady-state combined molar AUC of mobocertinib and its active metabolites by 92%.
Coadministration of EXKIVITY with multiple doses of efavirenz (a moderate CYP3A inducer) is predicted to decrease the steady-state combined molar AUC of mobocertinib and its active metabolites by 58%.
Effect of Mobocertinib on CYP3A Substrates: Coadministration of EXKIVITY 160 mg once daily with oral or intravenous midazolam (a CYP3A substrate) decreased the AUC of midazolam by 32% and 16%, respectively.
In Vitro Studies
CYP Enzymes: Mobocertinib, AP32960, and AP32914 do not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, or 2D6 at clinically relevant concentrations.
Transporter Systems: Mobocertinib is an inhibitor of P-gp and BCRP. At clinically relevant concentrations, mobocertinib does not inhibit BSEP, MATE1, MATE2-K, MRP2, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, or OCT2.
Mobocertinib is a substrate of P-gp. Mobocertinib is not a substrate of BCRP, OATP1B1, and OATP1B3.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were not performed with mobocertinib. Mobocertinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and did not induce chromosomal aberrations in an in vitro chromosome aberration assay in human peripheral blood lymphocytes. Mobocertinib was not clastogenic in an in vivo bone marrow micronucleus test in rats.
Fertility, early embryonic development, and pre- and post-natal toxicology studies were not conducted with mobocertinib; however, in 4- and 13-week repeat-dose toxicology studies in rats and dogs, there were generally reversible changes that included decreases in organ weights affecting multiple reproductive organs (including ovaries, seminal vesicle/prostate gland, and/or uterus) at exposures ≥0.3 times the AUC observed at the recommended clinical dose of 160 mg once daily, as well as microscopic changes of decreased epithelial thickness/inflammation of the cervix/vagina and atrophy of the uterus, prostate gland, or mammary gland (males only) at exposures ≥0.2 times the AUC at the 160 mg once daily clinical dose in rats and/or dogs. Based on these findings, mobocertinib may impair fertility in males and females of reproductive potential. These effects may be reversible.
13.2 Animal Toxicology and/or Pharmacology
In rats, mobocertinib administration resulted in histological findings of decreased corneal epithelial thickness in the 4- and 13-week repeat-dose toxicology studies at doses ≥0.8 times the human exposure (AUC) at the 160 mg once daily clinical dose. In the 4-week repeat-dose study in dogs, mobocertinib administration resulted in discharge from the eye, sclera injection, partial or complete closure of the eye and histological findings of corneal epithelial atrophy at doses ≥0.3 times the AUC at the 160 mg once daily clinical dose. In the 13-week repeat-dose study in dogs, mobocertinib administration resulted in discharge, conjunctival hyperemia, and corneal opacity correlating histologically with decreased corneal epithelial thickness at doses ≥0.2 times the AUC at the 160 mg once daily clinical dose. The clinical relevance of these findings is unknown.
-
14 CLINICAL STUDIES
The efficacy of EXKIVITY was evaluated in a pooled subset of patients with EGFR exon 20 insertion mutation-positive metastatic or locally advanced NSCLC whose disease had progressed on or after platinum-based chemotherapy enrolled in an international, open-label, multicohort clinical trial (AP32788-15-101, NCT02716116). Patients had histologically or cytologically confirmed locally advanced or metastatic disease (Stage IIIB or IV) and a documented EGFR exon 20 insertion mutation based on local testing. Patients received EXKIVITY at a dose of 160 mg once daily until disease progression or intolerable toxicity.
In the efficacy population, EGFR exon 20 insertion mutation status was determined by prospective local testing using samples from tumor tissue (87%), plasma (5%), or other specimens such as pleural fluid (8%). Of the 114 patients with EGFR exon 20 insertion mutations, 70% of patient tissue samples were tested retrospectively using Life Technologies Corporation Oncomine Dx™ Target Test. While 75% of patients were positive for EGFR exon 20 insertion mutation, 14% did not have an EGFR exon 20 insertion mutation identified, and 11% did not generate reportable results.
The efficacy population consisted of 114 patients and had the following demographic characteristics: the median age was 60 years (range: 27 to 84 years); 66% were female; 60% were Asian, 37% were White, and 3% were Black; 71% had never smoked; at baseline, 75% had Eastern Cooperative Oncology Group (ECOG) performance status 1. At baseline, 99% of patients had metastatic disease, 98% of patients had adenocarcinoma histology and 35% of patients had brain metastases. The median number of prior therapies was 2 (range: 1 to 7) and 43% percent had received prior immunotherapy.
The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by blinded independent central review (BICR). Additional efficacy outcome measures included duration of response (DOR) by BICR.
Efficacy results are summarized in Table 5.
Table 5: Efficacy Results in Patients with EGFR Exon 20 Insertion Mutation-Positive NSCLC Whose Disease Has Progressed on or after Platinum-Based Chemotherapy in Study AP32788-15-101 EXKIVITY
(n=114)Overall Response Rate (ORR)* (95% CI) 28% (20, 37)† Duration of Response (DOR) Median (months)‡, (95% CI) 17.5 (7.4, 20.3) Patients with DOR ≥6 months§ 59% Investigator-assessed ORR was 35% (95% CI: 26, 45) with a median DOR of 11.2 months (63% of these patients had observed responses lasting longer than 6 months).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
QTc Interval Prolongation and Torsades de Pointes
Inform patients of the risk of QTc prolongation. Symptoms that may be indicative of significant QTc prolongation include dizziness, lightheadedness, and syncope. Advise patients to report these symptoms and to inform their healthcare provider about the use of any heart medications [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (ILD)/Pneumonitis
Inform patients of the risks of severe or fatal ILD/pneumonitis. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms such as cough, shortness of breath or chest pain [see Warnings and Precautions (5.2)].
Cardiac Toxicity
Inform patients of the risk of heart failure. Advise patients to contact their healthcare provider immediately if they experience any signs or symptoms of heart failure such as palpitations, shortness of breath, chest pain, and syncope [see Warnings and Precautions (5.3)].
Diarrhea
Inform patients that EXKIVITY may cause diarrhea, which may be severe in some cases and should be treated promptly. Advise patients to have antidiarrheal medicine readily available and promptly start antidiarrheal treatment (e.g., loperamide), increase oral fluids and electrolyte intake, and contact their healthcare provider if diarrhea occurs [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective non-hormonal contraception during treatment with EXKIVITY and for 1 month after the last dose [see Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with EXKIVITY and for 1 week after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with EXKIVITY and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential that EXKIVITY may impair fertility [see Use in Specific Populations (8.3)].
Drug Interactions
Advise patients to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)]. Inform patients to avoid grapefruit or grapefruit juice while taking EXKIVITY.
Missed Dose
Advise patients that if a dose of EXKIVITY is missed by 6 hours or if vomiting occurs, resume treatment as prescribed the next day [see Dosage and Administration (2.3)].
-
SPL UNCLASSIFIED SECTION
Distributed by:
Takeda Pharmaceuticals America, Inc.
Lexington, MA 02421EXKIVITY® and the EXKIVITY Logo® are registered trademarks of Takeda Pharmaceuticals International AG. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
©2023 Takeda Pharmaceuticals U.S.A., Inc. All right reserved.
EXK366 R3
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: September 2023 PATIENT INFORMATION
EXKIVITY (ex ki vi tee)
(mobocertinib)
capsulesWhat is the most important information I should know about EXKIVITY?
EXKIVITY may cause serious side effects, including:- Changes in the electrical activity of your heart called QTc prolongation and Torsades de Pointes. QTc prolongation can cause irregular heartbeats that can be life-threatening and may lead to death. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) and do blood tests to check your electrolytes before starting and during treatment with EXKIVITY. Tell your healthcare provider right away if you feel dizzy, lightheaded, faint or have an irregular heartbeat.
What is EXKIVITY?
EXKIVITY is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC):- that has spread to other parts of the body (metastatic) and cannot be removed by surgery, and
- has a certain abnormal epidermal growth factor receptor (EGFR) gene, and
- whose disease has worsened while on or after chemotherapy that contains platinum
It is not known if EXKIVITY is safe and effective in children.Before taking EXKIVITY, tell your healthcare provider about all of your medical conditions, including if you: - have heart problems, including a condition called long QTc syndrome
- have problems with your electrolytes, such as sodium, potassium, calcium or magnesium
- have lung or breathing problems other than lung cancer
- have kidney problems
- are pregnant or plan to become pregnant. EXKIVITY can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with EXKIVITY.
- You should use an effective form of non-hormonal birth control during treatment and for 1 month after your last dose of EXKIVITY.
- Birth control pills (oral contraceptives) and other hormonal forms of birth control may not work as well during treatment with EXKIVITY.
- Talk to your healthcare provider about birth control methods that might be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with EXKIVITY.
- You should use effective birth control during treatment and for 1 week after your last dose of EXKIVITY.
- are breastfeeding or plan to breastfeed. It is not known if EXKIVITY passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of EXKIVITY.
EXKIVITY and other medicines may affect each other causing serious side effects.How should I take EXKIVITY? - Take EXKIVITY exactly as your healthcare provider tells you to take it.
- Take your prescribed dose of EXKIVITY 1 time each day, at the same time each day.
- Take EXKIVITY with or without food.
- Swallow EXKIVITY capsules whole. Do not open, chew, or dissolve the contents of the capsules.
- Do not change your dose or stop taking EXKIVITY unless your healthcare provider tells you to.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with EXKIVITY if you develop certain side effects.
- If you miss a dose of EXKIVITY, and it has been more than 6 hours, skip the dose and take your next dose at your regularly scheduled time the next day.
- If you vomit a dose of EXKIVITY, do not take an extra dose. Take your next dose at your regularly scheduled time the next day.
What should I avoid while taking EXKIVITY? - Avoid eating grapefruit or drinking grapefruit juice during treatment with EXKIVITY. Grapefruit may increase the amount of EXKIVITY in your blood.
What are the possible side effects of EXKIVITY?
EXKIVITY may cause serious side effects, including:
See "What is the most important information I should know about EXKIVITY?"- Lung problems. EXKIVITY may cause severe lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you develop any new or worsening symptoms, including trouble breathing or shortness of breath, cough, chest pain, or fever.
- Heart problems, including heart failure. EXKIVITY may cause heart problems that may lead to death. Your healthcare provider should check your heart function before you start and during treatment with EXKIVITY. Tell your healthcare provider right away if you have any signs or symptoms of a heart problem, including feeling like your heart is pounding or racing, shortness of breath, chest pain, swelling of your ankles and feet, or feeling faint.
- Diarrhea. Diarrhea is common during treatment with EXKIVITY, and may sometimes be severe. Diarrhea can cause you to lose too much body fluid (dehydration) and kidney problems. Your healthcare provider may tell you to start drinking more fluids and electrolytes to replace body salts or start taking your antidiarrheal medicines. Tell your healthcare provider right away if you have any loose stools or have stools more often than is normal for you.
- diarrhea
- rash
- mouth sores
- vomiting
- decreased appetite
- infection of skin around nails
- nausea
- muscle or bone pain
- dry skin
- tiredness
- cough
- itching
- decreased weight
The most common severe abnormal blood test results with EXKIVITY include: - decreased white blood cell counts
- increased amylase
- increased lipase
- decreased potassium
- decreased red blood cell counts
- increased creatinine
- decreased magnesium
- increased alanine aminotransferase
EXKIVITY may affect fertility in females and males, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of EXKIVITY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store EXKIVITY? - Store EXKIVITY at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of EXKIVITY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EXKIVITY for a condition for which it was not prescribed. Do not give EXKIVITY to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about EXKIVITY that is written for health professionals.What are the ingredients in EXKIVITY?
Active ingredient: mobocertinib
Inactive ingredients: None
Capsule shells: gelatin and titanium dioxide. The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water.
Distributed by: Takeda Pharmaceuticals America, Inc., Lexington, MA 02421.
EXKIVITY® and the EXKIVITY Logo® are registered trademarks of Takeda Pharmaceuticals International AG. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
©2023 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
For more information, go to www.EXKIVITY.com or call 1-844-217-6468. EXK366 R2 - PRINCIPAL DISPLAY PANEL - 40 mg Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
EXKIVITY
mobocertinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63020-040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mobocertinib (UNII: 39HBQ4A67L) (Mobocertinib - UNII:39HBQ4A67L) Mobocertinib 40 mg Product Characteristics Color WHITE Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 40mg;MB788 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63020-040-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2021 2 NDC:63020-040-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215310 09/15/2021 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations AndersonBrecon Inc. 053217022 PACK(63020-040) , LABEL(63020-040) Establishment Name Address ID/FEI Business Operations Takeda Ireland Limited 988980314 PACK(63020-040) , LABEL(63020-040) Establishment Name Address ID/FEI Business Operations Lonza Tampa LLC 628112240 MANUFACTURE(63020-040) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, Inc. 069777290 ANALYSIS(63020-040) Establishment Name Address ID/FEI Business Operations Ash Stevens LLC 049265333 API MANUFACTURE(63020-040) Establishment Name Address ID/FEI Business Operations Catalent Micron Technologies, Inc. 015966157 PARTICLE SIZE REDUCTION(63020-040)


