Label: DOPTELET- avatrombopag maleate tablet, film coated
- NDC Code(s): 71369-020-10, 71369-020-11, 71369-020-15, 71369-020-16, view more
- Packager: AkaRx, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOPTELET safely and effectively. See full prescribing information for DOPTELET. DOPTELET® (avatrombopag) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
1.1 - Treatment of Thrombocytopenia in Patients with Chronic Liver Disease (CLD) DOPTELET is indicated for the treatment of thrombocytopenia in adult patients with chronic liver ...
-
2
DOSAGE AND ADMINISTRATION
2.1 - Recommended Dosage for Patients with Chronic Liver Disease - Begin DOPTELET dosing 10 to 13 days prior to the scheduled procedure. The recommended daily dose of DOPTELET is based ...
-
3
DOSAGE FORMS AND STRENGTHS
Tablets: 20 mg as round, biconvex, yellow, film-coated tablets debossed with “AVA” on one side and “20” on the other side.
-
4
CONTRAINDICATIONS
None.
-
5
WARNINGS AND PRECAUTIONS
5.1 - Thrombotic/Thromboembolic Complications - DOPTELET is a thrombopoietin (TPO) receptor agonist and TPO receptor agonists have been associated with thrombotic and thromboembolic ...
-
6
ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in detail in other sections of the labeling: Thrombotic/Thromboembolic Complications [see Warnings and Precautions ...
-
7
DRUG INTERACTIONS
7.1 - Effect of Other Drugs on DOPTELET in Patients with Chronic Immune Thrombocytopenia - Moderate or Strong Dual Inhibitors of CYP2C9 and CYP3A4 - Concomitant use with a moderate or ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - Based on findings from animal reproduction studies, DOPTELET may cause fetal harm when administered to a pregnant woman (see Data). The available data on ...
-
10
OVERDOSAGE
In the event of overdose, platelet count may increase excessively and result in thrombotic or thromboembolic complications. Closely monitor the patient and platelet count. Treat thrombotic ...
-
11
DESCRIPTION
The active ingredient in DOPTELET is avatrombopag maleate, a thrombopoietin receptor agonist. The chemical name of avatrombopag maleate is 4-piperidinecarboxylic acid ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Avatrombopag is an orally bioavailable, small molecule TPO receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - In two-year carcinogenicity studies, avatrombopag was administered orally at doses of 20, 60, and 160 mg/kg/day in mice and ...
-
14
CLINICAL STUDIES
14.1 - Patients with Chronic Liver Disease - The efficacy of DOPTELET for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
DOPTELET 20 mg tablets are supplied as round, biconvex, yellow, film-coated tablets, and debossed with “AVA” on one side and “20” on the other side. How SuppliedCarton NDCBlister Card ...
-
17
PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information). Prior to treatment, patients should fully understand and be informed of the following risks and ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - DOPTELET® (dop-TEL-et) (avatrombopag) tablets - What is DOPTELET? DOPTELET is a prescription medicine used to treat low blood platelet counts in adults ...
-
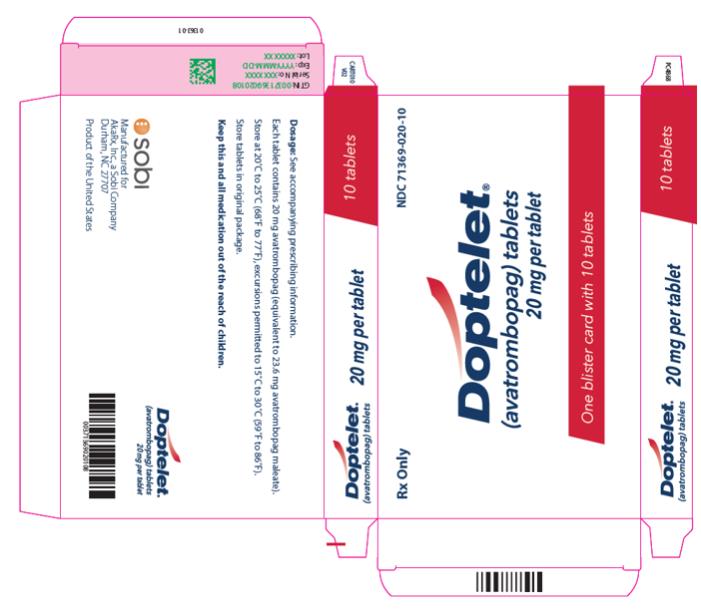
PRINCIPAL DISPLAY PANEL
NDC 71369-020-10 - 20 mg per tablet - Rx Only - Doptelet - one blister card with 10 tablets
-
PRINCIPAL DISPLAY PANEL
NDC 71369-020-15 - 15 mg per tablet - Rx Only - Doptelet - one blister card with 15 tablets
-
PRINCIPAL DISPLAY PANEL
NDC 71369-020-30 - 20 mg per tablet - Rx Only - Doptelet - Two blister cards with 15 tablets each (30 tablets)
-
INGREDIENTS AND APPEARANCEProduct Information




![The structural formula for DOPTELET is avatrombopag maleate, a thrombopoietin receptor agonist. The chemical name of avatrombopag maleate is 4-piperidinecarboxylic acid, 1-[3-chloro-5-[[[4-(4-chloro-2-thienyl)-5-(4-cyclohexyl-1-piperazinyl)-2-thiazolyl]amino]carbonyl]-2-pyridinyl]-, (2Z)-2-butenedioate (1:1). It has the molecular formula C29H34Cl2N6O3S2 · C4H4O4. The molecular weight is 765.73.](/dailymed/image.cfm?name=doptelet-01.jpg&setid=e2d5960d-6c18-46cc-86bd-089222b09852)