Label: XYWAV- calcium, magnesium, potassium, and sodium oxybates solution
- NDC Code(s): 68727-150-01
- Packager: Jazz Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: New Drug Application
Drug Label Information
Updated April 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XYWAV safely and effectively. See full prescribing information for XYWAV. XYWAV® (calcium, magnesium, potassium, and sodium ...These highlights do not include all the information needed to use XYWAV safely and effectively. See full prescribing information for XYWAV.
XYWAV® (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII
Initial U.S. Approval: 2002WARNING: CENTRAL NERVOUS SYSTEM (CNS) DEPRESSION and ABUSE AND MISUSE.
See full prescribing information for complete boxed warning.
Central Nervous System DepressionAbuse and Misuse
- •
- The active moiety of XYWAV is oxybate or gamma-hydroxybutyrate (GHB). Abuse or misuse of illicit GHB is associated with CNS adverse reactions, including seizure, respiratory depression, decreased consciousness, coma, and death (5.2, 9.2)
XYWAV is available only through a restricted program called the XYWAV and XYREM REMS (5.3)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
See Full Prescribing Information for complete dosing instructions (2.1-2.7).
Dosage for Adult Patients with Narcolepsy
- •
- Initiate dosage at 4.5 g per night orally, divided into two doses (2.1).
- •
- Titrate to effect in increments of up to 1.5 g per night per week (2.1).
- •
- Recommended dosage range: 6 g to 9 g per night orally, divided into two doses (2.1).
- •
- Doses may be divided equally or unequally and the first dose taken at bedtime and the second dose taken 2.5 to 4 hours later (2.1).
Dosage for Pediatric Patients with Narcolepsy (7 Years of Age and Older)
- •
- The recommended starting dosage, titration regimen, and maximum total nightly dosage are based on body weight (2.2).
Dosage for Adult Patients with Idiopathic Hypersomnia- •
- XYWAV can be administered as a twice or once nightly regimen in adults (2.3).
- •
- Twice nightly: Initiate dosage at 4.5 g or less per night orally, divided into two doses. Titrate to effect in increments of up to 1.5 g per night per week, up to 9 g total nightly dose (2.3).
- •
- Once nightly: Initiate dosage at 3 g or less per night orally, as one dose. Titrate to effect in increments of up to 1.5 g per night per week, up to 6 g total nightly dose (2.3).
Important Administration Information- •
- Administer XYWAV at least 2 hours after eating (2.4).
- •
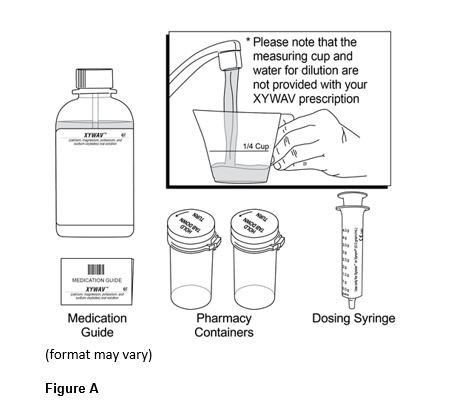
- Prepare XYWAV prior to bedtime; dilute with approximately ¼ cup of water in pharmacy-provided containers (2.4).
- •
- Take XYWAV while in bed and lie down after dosing (2.4).
For Patients Transitioning from Xyrem to XYWAV: Initiate at the same dose and regimen as Xyrem (gram for gram). Titrate as needed based on efficacy and tolerability (2.5).
Patients with Hepatic ImpairmentRecommended starting dosage is one-half of the original dosage per night administered orally, divided into two doses (2.6).
DOSAGE FORMS AND STRENGTHS
Oral solution: 0.5 g/mL total salts (equivalent to 0.413 g/mL of oxybate) (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- CNS depression: Use caution when considering the concurrent use of XYWAV with other CNS depressants (5.1).
- •
- Caution patients against hazardous activities requiring complete mental alertness or motor coordination within the first 6 hours of dosing or after first initiating treatment until certain that XYWAV does not affect them adversely (5.1).
- •
- Depression and suicidality: Monitor patients for emergent or increased depression and suicidality (5.5).
- •
- Confusion/Anxiety: Monitor for impaired motor/cognitive function (5.6).
- •
- Parasomnias: Evaluate episodes of sleepwalking (5.7).
ADVERSE REACTIONS
Most common adverse reactions in adults with narcolepsy or IH (≥5%) were nausea, headache, dizziness, anxiety, insomnia, decreased appetite, hyperhidrosis, vomiting, diarrhea, dry mouth, parasomnia, somnolence, fatigue, and tremor (6.1).
In a pediatric study with sodium oxybate (same active moiety as XYWAV), the most common adverse reactions (≥5%) were nausea, enuresis, vomiting, headache, weight decreased, decreased appetite, dizziness, and sleepwalking (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals, Inc. at 1-800-520-5568, or FDA at 1-800-FDA-1088 or www.fda.gov/Medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CENTRAL NERVOUS SYSTEM DEPRESSION
and ABUSE AND MISUSE.1 INDICATIONS AND USAGE
1.1 Narcolepsy
1.2 Idiopathic Hypersomnia
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information in Adult Patients with Narcolepsy
2.2 Dosing Information in Pediatric Patients with Narcolepsy
2.3 Dosing Information in Adult Patients with Idiopathic Hypersomnia (IH)
2.4 Important Administration Instructions for All Patients
2.5 Patients Transitioning from Xyrem to XYWAV
2.6 Dosage Modification in Patients with Hepatic Impairment
2.7 Dosage Adjustment with Co-administration of Divalproex Sodium
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Central Nervous System Depression
5.2 Abuse and Misuse
5.3 XYWAV and XYREM REMS
5.4 Respiratory Depression and Sleep-Disordered Breathing
5.5 Depression and Suicidality
5.6 Other Behavioral or Psychiatric Adverse Reactions
5.7 Parasomnias
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Alcohol, Sedative Hypnotics, and CNS Depressants
7.2 Divalproex Sodium
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Signs and Symptoms
10.3 Recommended Treatment of Overdose
10.4 Poison Control Center
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Cataplexy and Excessive Daytime Sleepiness (EDS) in Adult Narcolepsy
14.2 Cataplexy and Excessive Daytime Sleepiness in Pediatric Narcolepsy
14.3 Idiopathic Hypersomnia (IH) in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
16.3 Handling and Disposal
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CENTRAL NERVOUS SYSTEM DEPRESSION
and ABUSE AND MISUSE.- •
-
Central Nervous System Depression
XYWAV is a CNS depressant. Clinically significant respiratory depression and obtundation may occur in patients treated with XYWAV at recommended doses [see Warnings and Precautions (5.1, 5.4)]. Many patients who received XYWAV during clinical trials in narcolepsy and idiopathic hypersomnia were receiving central nervous system stimulants [see Clinical Studies (14.1, 14.2, 14.3)].
- •
-
Abuse and Misuse
The active moiety of XYWAV is oxybate or gamma-hydroxybutyrate (GHB). Abuse or misuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreases in the level of consciousness, coma, and death [see Warnings and Precautions (5.2)].
Because of the risks of CNS depression and abuse and misuse, XYWAV is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XYWAV and XYREM REMS [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE1.1 Narcolepsy - XYWAV is indicated for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy. 1.2 Idiopathic Hypersomnia ...
1.1 Narcolepsy
XYWAV is indicated for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
Close1.2 Idiopathic Hypersomnia
XYWAV is indicated for the treatment of idiopathic hypersomnia (IH) in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information in Adult Patients with Narcolepsy - The recommended starting dosage is 4.5 grams (g) per night administered orally, divided into two doses: 2.25 g at bedtime and 2.25 g ...
2.1 Dosing Information in Adult Patients with Narcolepsy
The recommended starting dosage is 4.5 grams (g) per night administered orally, divided into two doses: 2.25 g at bedtime and 2.25 g taken 2.5 to 4 hours later (see Table 1). Increase the dosage by up to 1.5 g per night per week (e.g., 0.75 g at bedtime and 0.75 g taken 2.5 to 4 hours later), to the recommended dosage range of 6 g to 9 g per night. The dosage may be gradually titrated based on efficacy and tolerability. Some patients may achieve better responses with unequal doses at bedtime and 2.5 to 4 hours later. Doses higher than 9 g per night have not been studied and ordinarily should not be administered.
Table 1: Recommended Adult XYWAV Dosage Regimen (g = grams) If a Patient’s Total Nightly Dosage Is:
Take at Bedtime:
Take 2.5 to 4 Hours Later:
4.5 g per night
2.25 g
2.25 g
6 g per night
3 g
3 g
7.5 g per night
3.75 g
3.75 g
9 g per night
4.5 g
4.5 g
Note: Some patients may achieve better responses with unequal nightly doses at bedtime and 2.5 to 4 hours later.
2.2 Dosing Information in Pediatric Patients with Narcolepsy
For pediatric patients 7 years of age and older, XYWAV is administered orally twice per night. The recommended starting pediatric dosage, titration regimen, and maximum total nightly dosage are based on patient weight, as specified in Table 2. The dosage may be gradually titrated based on efficacy and tolerability. Doses higher than 9 g per night have not been studied and ordinarily should not be administered.
Table 2: Recommended XYWAV Dosage for Patients 7 Years of Age and Older* Patient Weight
Initial Dosage
Maximum Weekly Dosage Increase
Maximum Recommended Dosage
Take at Bedtime:
Take 2.5 to 4 Hours Later:
Take at Bedtime:
Take 2.5 to 4 Hours Later:
Take at Bedtime:
Take 2.5 to 4 Hours Later:
<20 kg**
There is insufficient information to provide specific dosing recommendations for patients who weigh less than 20 kg.
20 kg to <30 kg
≤1 g
≤1 g
0.5 g
0.5 g
3 g
3 g
30 kg to <45 kg
≤1.5 g
≤1.5 g
0.5 g
0.5 g
3.75 g
3.75 g
≥45 kg
≤2.25 g
≤2.25 g
0.75 g
0.75 g
4.5 g
4.5 g
* For patients who sleep more than 8 hours per night, the first nightly dose of XYWAV may be given at bedtime or after an initial period of sleep.
** If XYWAV is used in patients 7 years of age and older who weigh less than 20 kg, a lower starting dosage, lower maximum weekly dosage increases, and lower total maximum nightly dosage should be considered.
Note: Some patients may achieve better responses with unequal nightly doses at bedtime and 2.5 to 4 hours later.2.3 Dosing Information in Adult Patients with Idiopathic Hypersomnia (IH)
The dosage and regimen of XYWAV should be individualized based on clinical presentation [see Clinical Studies (14.3)].
XYWAV can be administered as a twice nightly or once nightly regimen. The recommended starting dose, titration guidance, and maximum nightly doses appear in Table 3:
Table 3: Recommended Nightly Dosage in Adult Patients with IH Dosing Regimen
Starting Nightly Dose
Titration Increments
Maximum Total Nightly Dose
Twice nightly*,†
≤4.5 g per night divided into two doses (e.g., 2.25 g each)
≤1.5 g per night per week (divided into two doses)
9 g (divided into two doses)
Once nightly
≤3 g per night
≤1.5 g per night per week
6 g
* Some patients may achieve better responses with unequal nightly doses at bedtime and 2.5 to 4 hours later.
† The first dose should be taken at bedtime and the second dose taken 2.5 to 4 hours later.The increase in the total nightly dose should not exceed 1.5 g /week. During titration, the dosing regimen may be changed between twice nightly and once nightly, as needed based on efficacy and tolerability [see Clinical Studies (14.3)]. Doses higher than 9 g per night or single dose administrations higher than 6 g have not been studied and should not be administered.
2.4 Important Administration Instructions for All Patients
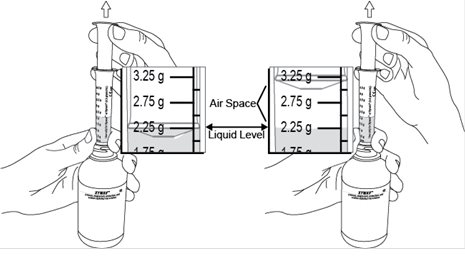
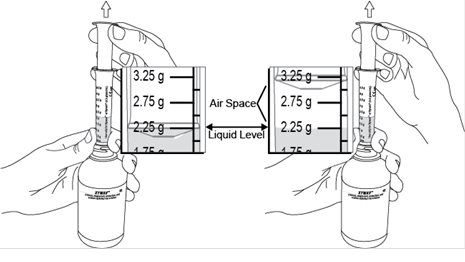
Administer XYWAV at least 2 hours after eating [see Clinical Pharmacology (12.3)]. Prepare all doses of XYWAV prior to bedtime. Prior to ingestion, each dose of XYWAV should be diluted with approximately ¼ cup (approximately 60 mL) of water in the empty pharmacy-provided containers. Solutions prepared following dilution should be consumed within 24 hours.
Patients should take each dose of XYWAV while in bed and lie down immediately after dosing, and remain in bed following ingestion of each dose. XYWAV may cause patients to fall asleep abruptly without first feeling drowsy [see Adverse Reactions (6.2)].
Patients will often fall asleep within 5 minutes of taking XYWAV, and will usually fall asleep within 15 minutes, though the time it takes any individual patient to fall asleep may vary from night to night.
If dosing twice nightly, patients may need to set an alarm to awaken for the second dose. If the second dose is missed, that dose should be skipped and XYWAV should not be taken again until the next night. Two XYWAV doses should never be taken at one time.
2.5 Patients Transitioning from Xyrem to XYWAV
On the first night of dosing with XYWAV, initiate treatment at the same dose (gram for gram) and regimen as Xyrem. Titrate as needed based on efficacy and tolerability [see Dosage and Administration (2.1)].
2.6 Dosage Modification in Patients with Hepatic Impairment
The recommended starting dosage in patients with hepatic impairment is one-half of the original dosage per night administered orally, divided into two doses [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Close2.7 Dosage Adjustment with Co-administration of Divalproex Sodium
When initiating divalproex sodium in patients taking a stable dosage of XYWAV, a reduction of the XYWAV dosage by at least 20% is recommended with initial concomitant use [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. When initiating XYWAV in patients already taking divalproex sodium, a lower starting dosage of XYWAV is recommended. Subsequently, the dosage of XYWAV can be adjusted based on individual clinical response and tolerability.
-
3 DOSAGE FORMS AND STRENGTHSXYWAV is a clear to slightly opalescent oral solution at a total salt concentration of 0.5 g per mL. Each mL contains 0.5 g of total salts present as 0.234 g calcium oxybate, 0.096 g magnesium ...
XYWAV is a clear to slightly opalescent oral solution at a total salt concentration of 0.5 g per mL. Each mL contains 0.5 g of total salts present as 0.234 g calcium oxybate, 0.096 g magnesium oxybate, 0.13 g potassium oxybate, and 0.04 g sodium oxybate (equivalent to 0.413 g total oxybate).
Close -
4 CONTRAINDICATIONSXYWAV is contraindicated for use in: • combination with sedative hypnotics [see Warnings and Precautions (5.1)]. • combination with alcohol [see Warnings and Precautions (5.1, 5.2)]. • patients ...
-
5 WARNINGS AND PRECAUTIONS5.1 Central Nervous System Depression - XYWAV is a central nervous system (CNS) depressant. Clinically significant respiratory depression and obtundation has occurred in adult patients taking ...
5.1 Central Nervous System Depression
XYWAV is a central nervous system (CNS) depressant. Clinically significant respiratory depression and obtundation has occurred in adult patients taking sodium oxybate (same active moiety as XYWAV) at recommended doses in clinical trials and may occur in patients treated with XYWAV at recommended doses. XYWAV is contraindicated in combination with alcohol and sedative hypnotics. The concurrent use of XYWAV with other CNS depressants, including but not limited to opioid analgesics, benzodiazepines, sedating antidepressants or antipsychotics, sedating anti-epileptic drugs, general anesthetics, muscle relaxants, and/or illicit CNS depressants, may increase the risk of respiratory depression, hypotension, profound sedation, syncope, and death.
If use of these CNS depressants in combination with XYWAV is required, dose reduction or discontinuation of one or more CNS depressants (including XYWAV) should be considered. In addition, if short-term use of an opioid (e.g., post- or perioperative) is required, interruption of treatment with XYWAV should be considered.
Healthcare providers should caution patients about operating hazardous machinery, including automobiles or airplanes, until they are reasonably certain that XYWAV does not affect them adversely (e.g., impair judgment, thinking, or motor skills). Patients should not engage in hazardous occupations or activities requiring complete mental alertness or motor coordination, such as operating machinery or a motor vehicle or flying an airplane, for at least 6 hours after taking XYWAV. Patients should be queried about CNS depression‐related events upon initiation of XYWAV therapy and periodically thereafter.
XYWAV is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.2 Abuse and Misuse
XYWAV is a Schedule III controlled substance. The active moiety of XYWAV is oxybate, also known as gamma-hydroxybutyrate (GHB), a Schedule I controlled substance. Abuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreases in the level of consciousness, coma, and death. The rapid onset of sedation, coupled with the amnestic features of GHB, particularly when combined with alcohol, has proven to be dangerous for the voluntary and involuntary user (e.g., assault victim). Because illicit use and abuse of GHB have been reported, healthcare providers should carefully evaluate patients for a history of drug abuse and follow them closely, particularly for signs of misuse or abuse of GHB (including but not limited to increase in size or frequency of dosing, drug-seeking behavior, feigned cataplexy) [see Drug Abuse and Dependence (9.2)]. If abuse is suspected, treatment with XYWAV should be discontinued.
XYWAV is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.3 XYWAV and XYREM REMS
XYWAV is available only through a restricted distribution program called the XYWAV and XYREM REMS because of the risks of central nervous system depression, and abuse and misuse [see Warnings and Precautions (5.1, 5.2)].
Notable requirements of the XYWAV and XYREM REMS include the following:
- •
- Healthcare Providers who prescribe XYWAV are specially certified
- •
- XYWAV will be dispensed only by the central pharmacy that is specially certified
- •
- XYWAV will be dispensed and shipped only to patients who are enrolled in the XYWAV and XYREM REMS with documentation of safe use.
Further information is available at www.XYWAVXYREMREMS.com or 1-866-997-3688.
5.4 Respiratory Depression and Sleep-Disordered Breathing
XYWAV may impair respiratory drive, especially in patients with compromised respiratory function. In overdoses of oxybate and with illicit use of GHB, life-threatening respiratory depression has been reported [see Overdosage (10)].
Increased apnea and reduced oxygenation may occur with XYWAV administration in adult and pediatric patients. A significant increase in the number of central apneas and clinically significant oxygen desaturation may occur in patients with obstructive sleep apnea treated with XYWAV.
In a study assessing the respiratory-depressant effects of Xyrem (same active moiety as XYWAV) at doses up to 9 g per night in 21 adult patients with narcolepsy, no dose-related changes in oxygen saturation were demonstrated in the group as a whole. One of the four patients with preexisting moderate-to-severe sleep apnea had significant worsening of the apnea/hypopnea index during treatment.
In a study assessing the effects of Xyrem 9 g per night in 50 adult patients with obstructive sleep apnea, Xyrem did not increase the severity of sleep‑disordered breathing and did not adversely affect the average duration and severity of oxygen desaturation overall. However, there was a significant increase in the number of central apneas in patients taking Xyrem, and clinically significant oxygen desaturation (≤55%) was measured in three patients (6%) after Xyrem administration, with one patient withdrawing from the study and two continuing after single brief instances of desaturation.
During polysomnographic evaluation (PSG), central sleep apnea and oxygen desaturation were observed in pediatric patients with narcolepsy treated with Xyrem.
Prescribers should be aware that increased central apneas and clinically relevant oxygen desaturation events have been observed with sodium oxybate administration in adult and pediatric patients.
In clinical trials of Xyrem in 128 adult patients with narcolepsy, two patients had profound CNS depression, which resolved after supportive respiratory intervention. Two other patients discontinued sodium oxybate because of severe difficulty breathing and an increase in obstructive sleep apnea. In two controlled trials assessing PSG measures in adult patients with narcolepsy, 40 of 477 patients were included with a baseline apnea/hypopnea index of 16 to 67 events per hour, indicative of mild to severe sleep-disordered breathing. None of the 40 patients had a clinically significant worsening of respiratory function, as measured by apnea/hypopnea index and pulse oximetry at doses of 4.5 g to 9 g per night.
Prescribers should be aware that sleep-related breathing disorders tend to be more prevalent in obese patients, in men, in postmenopausal women not on hormone replacement therapy, and among patients with narcolepsy.
5.5 Depression and Suicidality
Depression, and suicidal ideation and behavior can occur in patients treated with XYWAV.
In Study 1 [see Clinical Studies (14.1)], depression and depressed mood were reported in 3% and 4%, respectively, of patients treated with XYWAV. Two patients (1%) discontinued XYWAV because of depression, but in most cases, no change in XYWAV treatment was required.
In Study 2 [see Clinical Studies (14.3)], depression and depressed mood were reported in 1 patient (1%) and in 5 patients (3%), respectively, of patients treated with XYWAV, all of whom continued XYWAV treatment.
In clinical trials of Xyrem (same active moiety as XYWAV) in adult patients with narcolepsy (n=781), there were two suicides and two attempted suicides in patients treated with Xyrem, including three patients with a previous history of depressive psychiatric disorder. Of the two suicides, one patient used Xyrem in conjunction with other drugs. Xyrem was not involved in the second suicide. Adverse reactions of depression were reported by 7% of 781 patients treated with Xyrem, with four patients (<1%) discontinuing because of depression. In most cases, no change in Xyrem treatment was required. In a clinical trial with Xyrem in pediatric patients with narcolepsy (n=104), one patient experienced suicidal ideation and two patients reported depression while taking Xyrem.
The emergence of depression in patients treated with XYWAV requires careful and immediate evaluation. Patients with a previous history of a depressive illness and/or suicide attempt should be monitored carefully for the emergence of depressive symptoms while taking XYWAV.
5.6 Other Behavioral or Psychiatric Adverse Reactions
Other behavioral and psychiatric adverse reactions can occur in patients taking XYWAV.
In Study 1, confusion occurred in 1% of patients treated with XYWAV and anxiety occurred in 5% of patients treated with XYWAV. One patient experienced visual hallucinations and confusion after ingesting approximately 9 grams of XYWAV.
In Study 2, confusion occurred in 3% of patients treated with XYWAV, and anxiety occurred in 16% patients treated with XYWAV. One patient experienced visual hallucinations which led to discontinuation of XYWAV.
Other neuropsychiatric reactions reported in clinical trials of Xyrem (same active moiety as XYWAV) in adult patients with narcolepsy and in the postmarketing setting included hallucinations, paranoia, psychosis, aggression, and agitation.
In a pediatric clinical trial with Xyrem in patients with narcolepsy, neuropsychiatric reactions, including acute psychosis, confusion, and anxiety, were reported while taking Xyrem.
The emergence or increase in the occurrence of behavioral or psychiatric events in patients taking XYWAV should be carefully monitored.
Close5.7 Parasomnias
Parasomnias can occur in patients taking XYWAV.
In Study 1, parasomnias, including sleepwalking, were reported in 6% of patients treated with XYWAV.
In Study 2, parasomnias, including sleepwalking, were reported in 5% of patients treated with XYWAV.
In a clinical trial of Xyrem (same active moiety as XYWAV) in adult patients with narcolepsy, five instances of sleepwalking with potential injury or significant injury were reported. Parasomnias, including sleepwalking, also have been reported in a pediatric clinical trial with sodium oxybate and in postmarketing experience with sodium oxybate.
Episodes of sleepwalking should be fully evaluated and appropriate interventions considered.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions appear in other sections of the labeling: • CNS depression [see Warnings and Precautions (5.1)] • Abuse and Misuse [see Warnings and ...
The following clinically significant adverse reactions appear in other sections of the labeling:
- •
- CNS depression [see Warnings and Precautions (5.1)]
- •
- Abuse and Misuse [see Warnings and Precautions (5.2)]
- •
- Respiratory Depression and Sleep-Disordered Breathing [see Warnings and Precautions (5.4)]
- •
- Depression and Suicidality [see Warnings and Precautions (5.5)]
- •
- Other Behavioral or Psychiatric Adverse Reactions [see Warnings and Precautions (5.6)]
- •
- Parasomnias [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adult Patients with Narcolepsy
The safety of XYWAV was evaluated in a 16‑week double-blind placebo-controlled randomized-withdrawal study in patients with narcolepsy with cataplexy (Study 1), which was followed by an open-label extension phase lasting 24 weeks [see Clinical Studies (14.1)]. Study 1 included an open‑label titration period (OL OTTP), a stable-dose period (SDP), and a double‑blind, placebo‑controlled, randomized-withdrawal period (DB RWP). A total of 201 patients, ages 18 to 70 years, received XYWAV at individually titrated doses for 14 weeks, followed by randomization to XYWAV or matching placebo for 2 weeks of treatment. The mean exposure to XYWAV during this study, including titration, the randomized withdrawal period, and the open-label extension, was 151 days. In patients who remained on treatment, adverse reactions tended to occur early and diminish over time.
Adverse Reactions Leading to Treatment Discontinuation in Study 1
In Study 1, 9 of 201 patients (4%) reported adverse reactions that led to withdrawal from the study (anxiety, decreased appetite, depressed mood, depression, fatigue, headache, irritability, nausea, pain in extremity, parasomnia, somnolence, and vomiting). The most common adverse reaction leading to discontinuation was nausea (1.5%). The majority of adverse reactions leading to discontinuation began during the first few weeks of treatment.
Commonly Observed Adverse Reactions
The most common adverse reactions in Study 1 (incidence ≥5% of XYWAV-treated patients) were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis, anxiety, and vomiting.
Adverse Reactions Occurring at an Incidence of 2% or Greater:
Table 4 lists adverse reactions observed in the open-label titration and stable dose periods of Study 1 that occurred at a frequency of 2% or greater in adult patients treated with XYWAV.
Table 4: Adverse Reactions Occurring in ≥2% of Adult Patients Treated with XYWAV in the Open-Label Titration and Stable Dose Periods in Study 1* Adverse Reaction
Open-Label Titration Period + Stable Dose Period
(14 weeks)
(N=201)
%Headache
20
Nausea
13
Dizziness
10
Decreased appetite
8
Parasomnia†
6
Diarrhea
6
Hyperhidrosis‡
6
Anxiety§
5
Vomiting
5
Fatigue¶
4
Dry mouth
4
Depressed mood
4
Enuresis
4
Irritability
3
Paresthesia
3
Depression
3
Tremor
3
Somnolence
2
Muscle spasms
2
*Adverse reactions related to XYWAV were reported less frequently, as an overall incidence, in patients on Xyrem at study entry than in Xyrem-naïve patients.
†Includes abnormal dreams, abnormal sleep-related event, rapid eye movements sleep abnormal, sleep paralysis, sleep talking, sleep terror, sleep-related eating disorder, somnambulism
‡Includes hyperhidrosis and night sweats
§Includes anxiety, agitation, panic attack, tension
¶Includes fatigue and asthenia
Pediatric Patients (7 Years of Age and Older) with Narcolepsy
In the pediatric clinical trial with Xyrem (same active moiety as XYWAV), 104 patients aged 7 to 17 years (37 patients aged 7 to 11 years; 67 patients aged 12 to 17 years) with narcolepsy received Xyrem for up to one year [see Clinical Studies (14.2)]. This study included an open-label safety continuation period in which eligible patients received Xyrem for up to an additional 2 years. The median and maximum exposure across the entire study were 371 and 987 days, respectively.
Adverse Reactions Leading to Treatment Discontinuation
In the pediatric clinical trial with Xyrem, 7 of 104 patients reported adverse reactions that led to withdrawal from the study (hallucination, tactile; suicidal ideation; weight decreased; sleep apnea syndrome; affect lability; anger, anxiety, depression; and headache).
Adverse Reactions in the Xyrem Pediatric Clinical Trial
The most common adverse reactions (≥5%) were nausea (20%), enuresis (19%), vomiting (18%), headache (17%), weight decreased (13%), decreased appetite (9%), dizziness (8%), and sleepwalking (6%).
Additional information regarding safety in pediatric patients appears in the following sections:
- •
- Respiratory Depression and Sleep-Disordered Breathing [see Warnings and Precautions (5.4)]
- •
- Depression and Suicidality [see Warnings and Precautions (5.5)]
- •
- Other Behavioral or Psychiatric Adverse Reactions [see Warnings and Precautions (5.6)]
- •
- Parasomnias [see Warnings and Precautions (5.7)]
The overall adverse reaction profile of Xyrem in the pediatric clinical trial was similar to that seen in the adult clinical trial program. The safety profile in pediatric patients with XYWAV is expected to be similar to that of adult patients treated with XYWAV and to that of pediatric patients treated with Xyrem.
Adult Patients with Idiopathic Hypersomnia
The safety of XYWAV was evaluated in a double-blind placebo-controlled randomized‑withdrawal study in patients with IH (Study 2). This study consisted of an open‑label titration period (OL OTTP) up to 14 weeks, a stable-dose period (SDP) for 2 weeks, a double‑blind, placebo‑controlled, randomized-withdrawal period (DB RWP) for 2 weeks, and an open-label extension period for 24 weeks (all study periods up to 42 weeks) [see Clinical Studies (14.3)]. The study was conducted in 154 adult male and female patients ages 19 to 75 years of age with IH. The mean exposure to XYWAV during this study, including titration, the randomized withdrawal period, and the open-label extension, was 204 days. In patients who remained on treatment, adverse reactions tended to occur early and diminish over time.
Adverse Reactions Leading to Treatment Discontinuation in Study 2
In Study 2, across all study periods (excluding placebo during the DB RWP) (up to 42 weeks), 17 of 154 patients (11%) reported adverse reactions that led to withdrawal from the study (anxiety, nausea, insomnia, vomiting, fatigue, feeling abnormal, fall, decreased appetite, dizziness, paresthesia, tremor, parasomnia, confusional state, hallucination visual, and irritability). The most common adverse reaction leading to discontinuation was anxiety (3.2%). The majority of adverse reactions leading to discontinuation began during the first few weeks of treatment.
Commonly Observed Adverse Reactions
The most common adverse reactions in Study 2 (incidence ≥5% of XYWAV‑treated patients) in addition to those observed in Study 1 as most common were insomnia, dry mouth, fatigue, somnolence, and tremor.
The safety profile observed in Study 2 was similar to that of Study 1. Adverse reactions occurring in ≥2% of patients treated with XYWAV in the open-label titration and stable dose periods in Study 2 are shown in Table 5:
Table 5: Adverse Reactions Occurring in ≥2% of Patients Treated with XYWAV in the Open-Label Titration and Stable Dose Periods in Study 2 Adverse Reaction
Open-Label Titration Period + Stable Dose Period
(up to 16 weeks)
(N=154)
%
Nausea
21
Headache
16
Anxiety*
12
Dizziness
12
Insomnia†
9
Hyperhidrosis‡
8
Decreased appetite
8
Vomiting
7
Dry mouth
6
Diarrhea
5
Fatigue§
5
Somnolence¶
5
Tremor
5
Parasomnia#
5
Balance disorder♠
3
Muscle spasms
3
Fall
3
Paresthesia
3
Snoring
3
Weight decreased
3
Bruxism
3
Confusional state
3
Depressed mood
3
Feeling drunk
3
Irritability
3
* includes anxiety, nervousness, and panic attack
† includes middle insomnia, initial insomnia, insomnia, and terminal insomnia
‡ includes hyperhidrosis and night sweats
§ includes fatigue and asthenia
¶ includes somnolence and sedation
# includes confusional arousal, sleep paralysis, nightmare, sleep talking, somnambulism, and
hypnopompic hallucination
♠ includes balance disorder and ataxia
Additional Adverse Reactions
Adverse reactions observed in clinical studies with Xyrem (≥2%), but not observed in Study 1 or Study 2 at a frequency of higher than 2%, and which may be relevant for XYWAV:
Pain, pain in extremity, cataplexy, disturbance in attention, sleep paralysis, and disorientation.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sodium oxybate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Arthralgia, fall*, fluid retention, hangover, hypersensitivity, hypertension, memory impairment, nocturia, and vision blurred.
- * The sudden onset of sleep in patients taking sodium oxybate, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization.
-
7 DRUG INTERACTIONS7.1 Alcohol, Sedative Hypnotics, and CNS Depressants - XYWAV is contraindicated for use in combination with alcohol or sedative hypnotics. Use of other CNS depressants may potentiate the ...
7.1 Alcohol, Sedative Hypnotics, and CNS Depressants
XYWAV is contraindicated for use in combination with alcohol or sedative hypnotics. Use of other CNS depressants may potentiate the CNS-depressant effects of XYWAV [see Warnings and Precautions (5.1)].
Close7.2 Divalproex Sodium
Concomitant use of sodium oxybate with divalproex sodium results in an increase in systemic exposure to GHB, which was shown to cause a greater impairment on some tests of attention and working memory in a clinical study [see Clinical Pharmacology (12.3)]. A similar increase in exposure is expected with concomitant use of XYWAV and divalproex sodium; therefore, an initial dose reduction of XYWAV is recommended when used concomitantly with divalproex sodium [see Dosage and Administration (2.7)]. Prescribers are advised to monitor patient response closely and adjust dose accordingly if concomitant use of XYWAV and divalproex sodium is warranted.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of XYWAV or sodium oxybate in pregnant women. Oral administration of sodium oxybate to ...
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of XYWAV or sodium oxybate in pregnant women. Oral administration of sodium oxybate to pregnant rats (0, 150, 350, or 1,000 mg/kg/day) or rabbits (0, 300, 600, or 1,200 mg/kg/day) throughout organogenesis produced no clear evidence of developmental toxicity; however, oral administration to rats throughout pregnancy and lactation resulted in increased stillbirths and decreased offspring postnatal viability and growth, at a clinically relevant dose [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Labor or Delivery
XYWAV has not been studied in labor or delivery. In obstetric anesthesia using an injectable formulation of sodium oxybate, newborns had stable cardiovascular and respiratory measures but were very sleepy, causing a slight decrease in Apgar scores. There was a fall in the rate of uterine contractions 20 minutes after injection. Placental transfer is rapid, and gamma-hydroxybutyrate (GHB) has been detected in newborns at delivery after intravenous administration of GHB to mothers. Subsequent effects of sodium oxybate on later growth, development, and maturation in humans are unknown.
Data
Animal Data
Oral administration of sodium oxybate to pregnant rats (0, 150, 350, or 1,000 mg/kg/day) or rabbits (0, 300, 600, or 1,200 mg/kg/day) throughout organogenesis produced no clear evidence of developmental toxicity. The highest doses of sodium oxybate tested in rats and rabbits were approximately 1 and 3 times, respectively, the maximum recommended human dose (MRHD) of 9 g per night on a body surface area (mg/m2) basis.
Additionally, oral administration of sodium oxybate (0, 150, 350, or 1,000 mg/kg/day) to rats throughout pregnancy and lactation resulted in increased stillbirths and decreased offspring postnatal viability and body weight gain at the highest dose tested. The no-effect dose for pre- and post-natal developmental toxicity in rats is less than the MRHD on a mg/m2 basis.
8.2 Lactation
Risk Summary
GHB is excreted in human milk after oral administration of sodium oxybate. There is insufficient information on the risk to a breastfed infant, and there is insufficient information on milk production in nursing mothers. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XYWAV and any potential adverse effects on the breastfed infant from XYWAV or from the underlying maternal condition.
8.4 Pediatric Use
Narcolepsy
The safety and effectiveness of XYWAV for the treatment of cataplexy or excessive daytime sleepiness in pediatric patients 7 years of age and older with narcolepsy have been established. XYWAV has not been studied in a pediatric clinical trial. Use of XYWAV in pediatric patients 7 years of age and older with narcolepsy is supported by evidence from an adequate and well-controlled study of sodium oxybate in pediatric patients 7 to 17 years of age, a study in adults showing a treatment effect of XYWAV similar to that observed with sodium oxybate, pharmacokinetic data of sodium oxybate from adult and pediatric patients, and pharmacokinetic data of XYWAV from healthy adult volunteers [see Adverse Reactions (6.1) and Clinical Studies (14.1, 14.2)].
In the pediatric clinical trial with sodium oxybate administration in patients with narcolepsy, serious adverse reactions of central sleep apnea and oxygen desaturation documented by polysomnography evaluation; depression; suicidal ideation; neuropsychiatric reactions including acute psychosis, confusion, and anxiety; and parasomnias, including sleepwalking, have been reported [see Warnings and Precautions (5.4, 5.5, 5.6, 5.7) and Adverse Reactions (6.1)].
Safety and effectiveness of XYWAV for the treatment of cataplexy or excessive daytime sleepiness in pediatric patients below the age of 7 years have not been established.
Idiopathic Hypersomnia
Safety and effectiveness of XYWAV for the treatment of idiopathic hypersomnia in pediatric patients have not been established.
Juvenile Animal Toxicity Data
In a study in which sodium oxybate (0, 100, 300, or 900 mg/kg/day) was orally administered to rats during the juvenile period of development (postnatal days 21 through 90), mortality was observed at the two highest doses tested. Deaths occurred during the first week of dosing and were associated with clinical signs (including decreased activity and respiratory rate) consistent with the pharmacological effects of the drug. Reduced body weight gain in males and females and delayed sexual maturation in males were observed at the highest dose tested. The no-effect dose for adverse effects in juvenile rats is associated with plasma exposures (AUC) less than that at the maximum recommended human dose (9 g/night).
8.5 Geriatric Use
Clinical studies of XYWAV or Xyrem in patients with narcolepsy or IH did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
In clinical studies of sodium oxybate in another population, 39 (5%) of 874 patients were 65 years or older. Discontinuations of treatment due to adverse reactions were increased in the elderly compared to younger adults (21% vs. 19%). Frequency of headaches was markedly increased in the elderly (39% vs. 19%). The most common adverse reactions were similar in both age categories. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Close8.6 Hepatic Impairment
Because of an increase in exposure to XYWAV, the starting dose should be reduced by half in patients with hepatic impairment [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - XYWAV is a Schedule III controlled substance under the Federal Controlled Substances Act. Non-medical use of XYWAV could lead to penalties assessed under the higher ...
9.1 Controlled Substance
XYWAV is a Schedule III controlled substance under the Federal Controlled Substances Act. Non-medical use of XYWAV could lead to penalties assessed under the higher Schedule I controls.
9.2 Abuse
The active moiety of XYWAV, oxybate, produces dose-dependent central nervous system effects, including hypnotic and positive subjective reinforcing effects. The onset of effect is rapid, enhancing its potential for abuse or misuse.
Drug abuse is the intentional non-therapeutic use of a drug product or substance, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug misuse and abuse may occur with or without progression to addiction. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
The rapid onset of sedation, coupled with the amnestic features of GHB, particularly when combined with alcohol, has proven to be dangerous for the voluntary and involuntary user (e.g., assault victim).
Illicit GHB is abused in social settings primarily by young adults. Some of the doses estimated to be abused are in a similar dosage range to that used for treatment of patients with cataplexy. GHB has some commonalities with ethanol over a limited dose range, and some cross tolerance with ethanol has been reported as well. Cases of severe dependence and craving for GHB have been reported when the drug is taken around the clock. Patterns of abuse indicative of dependence include: 1) the use of increasingly large doses, 2) increased frequency of use, and 3) continued use despite adverse consequences.
Because illicit use and abuse of GHB have been reported, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of GHB (e.g., increase in size or frequency of dosing, drug-seeking behavior, feigned cataplexy). Dispose of XYWAV according to state and federal regulations. It is safe to dispose of XYWAV down the sanitary sewer.
Close9.3 Dependence
Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. There have been case reports of withdrawal, ranging from mild to severe, following discontinuation of illicit use of GHB at frequent repeated doses (18 g to 250 g per day) in excess of the recommended dosage range. Signs and symptoms of GHB withdrawal following abrupt discontinuation included insomnia, restlessness, anxiety, psychosis, lethargy, nausea, tremor, sweating, muscle cramps, tachycardia, headache, dizziness, rebound fatigue and sleepiness, confusion, and, particularly in the case of severe withdrawal, visual hallucinations, agitation, and delirium. These symptoms generally abated in 3 to 14 days. In cases of severe withdrawal, hospitalization may be required. In the clinical trial experience with Xyrem in narcolepsy/cataplexy patients at recommended doses, two patients reported anxiety and one reported insomnia following abrupt discontinuation at the termination of the clinical trial; in the two patients with anxiety, the frequency of cataplexy had increased markedly at the same time. In the XYWAV clinical trial in adult narcolepsy/cataplexy patients at recommended doses, one patient reported insomnia following abrupt discontinuation of XYWAV. In the XYWAV clinical trial in adult idiopathic hypersomnia patients at recommended doses, six patients reported insomnia, two patients reported early insomnia, and one patient reported visual and auditory hallucinations following abrupt discontinuation of XYWAV.
Tolerance
Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to XYWAV has not been systematically studied in controlled clinical trials. There have been some case reports of symptoms of tolerance developing after illicit use at dosages far in excess of the recommended XYWAV dosage regimen. Clinical studies of sodium oxybate in the treatment of alcohol withdrawal suggest a potential cross-tolerance with alcohol. The safety and effectiveness of XYWAV in the treatment of alcohol withdrawal have not been established.
-
10 OVERDOSAGE10.1 Human Experience - Information regarding overdose with XYWAV is derived largely from reports in the medical literature that describe symptoms and signs in individuals who have ingested GHB ...
10.1 Human Experience
Information regarding overdose with XYWAV is derived largely from reports in the medical literature that describe symptoms and signs in individuals who have ingested GHB illicitly. In these circumstances the co-ingestion of other drugs and alcohol was common, and may have influenced the presentation and severity of clinical manifestations of overdose.
In adult clinical trials with Xyrem (same active moiety as XYWAV), two cases of overdose were reported. In the first case, an estimated dose of 150 g, more than 15 times the maximum recommended dose, caused a patient to be unresponsive with brief periods of apnea and to be incontinent of urine and feces. This individual recovered without sequelae. In the second case, death was reported following a multiple drug overdose consisting of Xyrem and numerous other drugs. No cases of overdose (greater than 9 g) with XYWAV were reported in the XYWAV clinical trials.
10.2 Signs and Symptoms
Information about signs and symptoms associated with overdosage with XYWAV derives from reports of illicit use of GHB. Patient presentation following overdose is influenced by the dose ingested, the time since ingestion, the co-ingestion of other drugs and alcohol, and the fed or fasted state. Patients have exhibited varying degrees of depressed consciousness that may fluctuate rapidly between a confusional, agitated combative state with ataxia and coma. Emesis (even when obtunded), diaphoresis, headache, and impaired psychomotor skills have been observed. No typical pupillary changes have been described to assist in diagnosis; pupillary reactivity to light is maintained. Blurred vision has been reported. An increasing depth of coma and acidosis have been observed at higher doses. Myoclonus and tonic-clonic seizures have been reported. Respiration may be unaffected or compromised in rate and depth. Cheyne-Stokes respiration and apnea have been observed. Bradycardia and hypothermia may accompany unconsciousness, as well as muscular hypotonia, but tendon reflexes remain intact.
10.3 Recommended Treatment of Overdose
General symptomatic and supportive care should be instituted immediately, and gastric decontamination may be considered if co-ingestants are suspected. Because emesis may occur in the presence of obtundation, appropriate posture (left lateral recumbent position) and protection of the airway by intubation may be warranted. Although the gag reflex may be absent in deeply comatose patients, even unconscious patients may become combative to intubation, and rapid-sequence induction (without the use of sedative) should be considered. Vital signs and consciousness should be closely monitored. The bradycardia reported with GHB overdose has been responsive to atropine intravenous administration. No reversal of the central depressant effects of XYWAV can be expected from naloxone or flumazenil administration. The use of hemodialysis and other forms of extracorporeal drug removal have not been studied in GHB overdose, but have been reported in cases of acidosis associated with GHB ingestions of 125 g or greater; however, due to the rapid metabolism of oxybate, these measures may not be warranted.
Close10.4 Poison Control Center
As with the management of all cases of drug overdosage, the possibility of multiple drug ingestion should be considered. The healthcare provider is encouraged to collect urine and blood samples for routine toxicologic screening, and to consult with a regional poison control center (1-800-222-1222) for current treatment recommendations.
-
11 DESCRIPTIONXYWAV oral solution contains oxybate, a CNS depressant. The chemical name of oxybate is gamma-hydroxybutyrate (GHB). XYWAV contains a mixture of calcium oxybate, magnesium oxybate, potassium ...
XYWAV oral solution contains oxybate, a CNS depressant. The chemical name of oxybate is gamma-hydroxybutyrate (GHB). XYWAV contains a mixture of calcium oxybate, magnesium oxybate, potassium oxybate, and sodium oxybate equivalent to 0.5 g/mL, which corresponds to 0.413 g/mL oxybate.
Each mL of XYWAV contains: 0.234 g calcium oxybate, Ca(C4H7O3)2; 0.096 g magnesium oxybate, Mg(C4H7O3)2; 0.13 g potassium oxybate, K(C4H7O3); and 0.04 g sodium oxybate, Na(C4H7O3) in dissociated form in the solution. The molecular weights of each are as follows: calcium oxybate is 246.3, magnesium oxybate is 230.5, potassium oxybate is 142.2, and sodium oxybate is 126.1.
The chemical structure is:
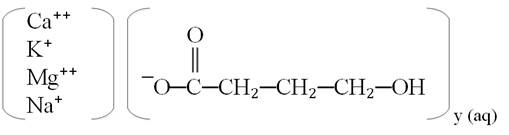
y=1 for Na+ and K+; y=2 for Mg2+ and Ca2+The inactive ingredients are purified water and sucralose.
Close
XYWAV contains no ingredient made from a gluten‑containing grain (wheat, barley, or rye). -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - XYWAV is a CNS depressant. The exact mechanism of action of XYWAV in the treatment of narcolepsy and idiopathic hypersomnia is unknown. XYWAV is a mixture of calcium ...
12.1 Mechanism of Action
XYWAV is a CNS depressant. The exact mechanism of action of XYWAV in the treatment of narcolepsy and idiopathic hypersomnia is unknown. XYWAV is a mixture of calcium oxybate, magnesium oxybate, potassium oxybate, and sodium oxybate (gamma-hydroxybutyrate). Gamma-hydroxybutyrate (GHB) is an endogenous compound and metabolite of the neurotransmitter GABA. It is hypothesized that the therapeutic effects of XYWAV are mediated through GABAB actions during sleep at noradrenergic and dopaminergic neurons, as well as at thalamocortical neurons.
Close12.3 Pharmacokinetics
Pharmacokinetics of GHB are nonlinear and are similar following single or repeat dosing. The pharmacokinetics of oxybate following administration of XYWAV are similar between healthy subjects and patients with narcolepsy or patients with idiopathic hypersomnia.
Absorption
Following oral administration of XYWAV, the average time to peak plasma concentration (Tmax) was about 1.3 hours in healthy adults in the fasted state.
Following oral administration of XYWAV, the plasma levels of GHB increased more than dose‑proportionally, with Cmax increasing approximately 2‐fold and AUC increasing 2.9-fold as the dose was doubled from 2.25 g to 4.5 g.
Effect of Food
Administration of XYWAV immediately after a high-fat meal resulted in a mean reduction in Cmax of GHB by 33%, and mean reduction in systemic exposure (AUC) by 16% [see Dosage and Administration (2.4)].
Distribution
GHB has an apparent volume of distribution averaging 190 mL/kg to 384 mL/kg. At GHB concentrations ranging from 3 mcg/mL to 300 mcg/mL. Less than 1% is bound to plasma proteins.
Elimination
Metabolism
Animal studies indicate that metabolism is the major elimination pathway for GHB, producing carbon dioxide and water via the tricarboxylic acid (Krebs) cycle and secondarily by beta-oxidation. The primary pathway involves a cytosolic NADP+-linked enzyme, GHB dehydrogenase, that catalyzes the conversion of GHB to succinic semialdehyde, which is then biotransformed to succinic acid by the enzyme succinic semialdehyde dehydrogenase. Succinic acid enters the Krebs cycle where it is metabolized to carbon dioxide and water. A second mitochondrial oxidoreductase enzyme, a transhydrogenase, also catalyzes the conversion to succinic semialdehyde in the presence of α-ketoglutarate. An alternate pathway of biotransformation involves β-oxidation via 3,4-dihydroxybutyrate to carbon dioxide and water. No active metabolites have been identified.
Excretion
The clearance of GHB is almost entirely by biotransformation to carbon dioxide, which is then eliminated by expiration. On average, less than 5% of unchanged drug appears in human urine within 6 to 8 hours after dosing. Fecal excretion is negligible. GHB has a mean terminal elimination half-life of 0.66 hours.
Specific Populations
Geriatric Patients
There is limited experience with sodium oxybate and no experience with XYWAV in the elderly. Results from a pharmacokinetic study (n=20) in another studied population indicate that the pharmacokinetic characteristics of GHB are consistent among younger (ages 48 to 64 years) and older (ages 65 to 75 years) adults.
Pediatric Patients
The pharmacokinetics of XYWAV has not been directly evaluated in pediatric patients.
The pharmacokinetics of sodium oxybate was evaluated in pediatric patients aged 7 to 17 years and demonstrated similar PK properties as adults. A population pharmacokinetic model was developed with sodium oxybate data from pediatric and adult patients and healthy volunteers and with XYWAV data from healthy adult volunteers. The population PK model analyses demonstrate that body weight is the major intrinsic factor affecting GHB pharmacokinetics following sodium oxybate or XYWAV dosing. Additionally, XYWAV has similar PK characteristics (more than dose proportionality) as sodium oxybate in pediatric patients, supporting the same dose regimen as sodium oxybate and 1-to-1 dose switch from sodium oxybate to XYWAV in pediatric patients.
Male and Female Patients
In a study of 18 female and 18 male healthy adult volunteers, no gender differences were detected in the pharmacokinetics of GHB following a single Xyrem oral dose of 4.5 g.
Racial or Ethnic Groups
There are insufficient data to evaluate any pharmacokinetic differences among races.
Patients with Renal Impairment
No pharmacokinetic study in patients with renal impairment has been conducted.
Patients with Hepatic Impairment
The pharmacokinetics of GHB in 16 cirrhotic patients, half without ascites (Child’s Class A) and half with ascites (Child’s Class C), were compared to the kinetics in 8 subjects with normal hepatic function after a single sodium oxybate oral dose of 25 mg/kg. AUC values were double in the cirrhotic patients, with apparent oral clearance reduced from 9.1 mL/min/kg in healthy adults to 4.5 and 4.1 mL/min/kg in Class A and Class C patients, respectively. Elimination half-life was significantly longer in Class C and Class A patients than in control patients (mean t1/2 of 59 and 32 minutes, respectively, versus 22 minutes). The starting dose of XYWAV should be reduced in patients with hepatic impairment [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Drug Interactions Studies
Studies in vitro with pooled human liver microsomes indicate that sodium oxybate does not significantly inhibit the activities of the human isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A up to the concentration of 3 mM (378 mcg/mL), a level considerably higher than levels achieved with recommended doses.
Drug interaction studies in healthy adults (age 18 to 50 years) were conducted with sodium oxybate and divalproex sodium, diclofenac, and ibuprofen.
- •
- Divalproex sodium: Co-administration of sodium oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with divalproex sodium (valproic acid, 1250 mg per day) increased mean systemic exposure to GHB as shown by AUC by approximately 25% (AUC ratio range of 0.8 to 1.7), while Cmax was comparable. Co-administration did not appear to affect the pharmacokinetics of valproic acid. A greater impairment on some tests of attention and working memory was observed with co-administration of both drugs than with either drug alone [see Drug Interactions (7.2) and Dosage and Administration (2.7)].
- •
- Diclofenac: Co-administration of sodium oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with diclofenac (50 mg/dose twice per day) showed no significant differences in systemic exposure to GHB. Co-administration did not appear to affect the pharmacokinetics of diclofenac.
- •
- Ibuprofen: Co-administration of sodium oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with ibuprofen (800 mg/dose four times per day also dosed four hours apart) resulted in comparable systemic exposure to GHB as shown by plasma Cmax and AUC values. Co-administration did not affect the pharmacokinetics of ibuprofen.
Drug interaction studies in healthy adults demonstrated no pharmacokinetic interactions between sodium oxybate and protriptyline hydrochloride, zolpidem tartrate, and modafinil. Also, there were no pharmacokinetic interactions with the alcohol dehydrogenase inhibitor fomepizole. However, pharmacodynamic interactions with these drugs cannot be ruled out. Alteration of gastric pH with omeprazole produced no significant change in the pharmacokinetics of GHB. In addition, drug interaction studies in healthy adults demonstrated no pharmacokinetic or clinically significant pharmacodynamic interactions between sodium oxybate and duloxetine HCl.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Administration of sodium oxybate to rats at oral doses of up to 1,000 mg/kg/day for 83 (males) or 104 (females) weeks ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Administration of sodium oxybate to rats at oral doses of up to 1,000 mg/kg/day for 83 (males) or 104 (females) weeks resulted in no increase in tumors. Plasma exposure (AUC) at the highest dose tested was 2 times that in humans at the maximum recommended human dose (MRHD) of 9 g per night.
The results of 2-year carcinogenicity studies in mouse and rat with gamma-butyrolactone, a compound that is metabolized to oxybate in vivo, showed no clear evidence of carcinogenic activity. The plasma AUCs of oxybate achieved at the highest doses tested in these studies were less than that in humans at the MRHD.
Mutagenesis
Sodium oxybate was negative in the in vitro bacterial gene mutation assay, an in vitro chromosomal aberration assay in mammalian cells, and in an in vivo rat micronucleus assay.
Impairment of Fertility
Oral administration of sodium oxybate (0, 150, 350, or 1,000 mg/kg/day) to male and female rats prior to and throughout mating and continuing in females through early gestation resulted in no adverse effects on fertility. The highest dose tested is approximately equal to the MRHD on a mg/m2 basis.
-
14 CLINICAL STUDIES14.1 Cataplexy and Excessive Daytime Sleepiness (EDS) in Adult Narcolepsy - Efficacy of XYWAV for the treatment of cataplexy and excessive daytime sleepiness in adult patients with narcolepsy was ...
14.1 Cataplexy and Excessive Daytime Sleepiness (EDS) in Adult Narcolepsy
Efficacy of XYWAV for the treatment of cataplexy and excessive daytime sleepiness in adult patients with narcolepsy was established in a double‑blind, placebo‑controlled, randomized‑withdrawal study (Study 1; NCT03030599). This study had two parts, consisting of the main study, followed by an optional 24-week open‑label extension (OLE). The main study consisted of a 12‑week open-label optimized treatment and titration period (OL OTTP), followed by a 2-week stable-dose period (SDP), and finally a 2‑week double-blind randomized-withdrawal period (DB RWP).
Study 1 enrolled 201 patients with narcolepsy with cataplexy, 18 to 70 years of age, with a baseline history of at least 14 cataplexy attacks in a typical 2-week period prior to any treatment for narcolepsy symptoms. Of the 201 patients, 134 were randomized 1:1 to continue treatment with XYWAV or to placebo in the 2‑week DB RWP. In the safety population, overall, the median age was 36.0 years (range: 18 to 70). The majority of subjects were female (61%), and most were white (88%) and not Hispanic or Latino (84%).
Patients entering the study were taking a stable dosage of 1) Xyrem only, 2) Xyrem + another anticataplectic, 3) a non-Xyrem anticataplectic, or 4) were cataplexy‑treatment naïve. Patients taking Xyrem at study entry were switched (at a gram for gram dose) from Xyrem to XYWAV for a minimum of 2 weeks and titrated, if needed, to a stable, tolerable, and effective dosage over 8 weeks. Most patients who switched from Xyrem to XYWAV (41/59; 69%) had no change in dosage from study entry to the stable dose period; 27% (16/59) had an increase in dosage, and 3% (2/59) had a decrease in dosage. Among patients whose dosage was changed, most changes were within one titration step (≤1.5 g). Patients not taking Xyrem at study entry were initiated at 4.5 g/night of XYWAV and titrated at a rate of 1 or 1.5 g/night/week to a tolerable dose of XYWAV. Patients taking an anticataplectic other than Xyrem were tapered off the non-Xyrem anticataplectic over 2 to 8 weeks. All patients continued to receive XYWAV only, for the treatment of cataplexy during the last 2 weeks of the OL OTTP.
CNS stimulants were allowed at entry, and approximately 59% of patients continued taking a stable dose of stimulant throughout the SDP and DB RWP.
The total nightly dose of XYWAV was administered in two equally divided doses in 90% (62/69) of patients. Unequal doses were administered in 10% (7/69) of patients treated with XYWAV.
The primary efficacy endpoint was the change in frequency of cataplexy attacks from the 2 weeks of the SDP to the 2 weeks of the DB RWP. The key secondary endpoint was the change in the Epworth Sleepiness Scale (ESS) score, as a measure of reduction in EDS from the end of the SDP to the end of the DB RWP.
Patients taking stable doses of XYWAV who discontinued XYWAV treatment and were randomized to placebo during the DB RWP experienced a significant worsening in the average weekly number of cataplexy attacks and in ESS score, compared with patients randomized to continue treatment with XYWAV (see Table 6).
Table 6: Mean and Median Number of Weekly Cataplexy Attacks and Epworth Sleepiness Scale (ESS) Average Weekly Number of Cataplexy Attacks
ESS SCORE
Placebo
(N=65)XYWAV
(N=69)Placebo
(N=65)XYWAV
(N=69)Baseline (2 Weeks of the Stable Dose Period)
Mean (SD)
7.2 (14.4)
8.9 (16.8)
12.6 (5.5)
13.6 (5.3)
Median
1.0
1.1
13.0
14.0
Change from Baseline (2 Weeks of the Stable Dose Period) to the 2 Weeks of the DB RWP
Change from End of Stable Dose Period to End of DB RWP
Mean (SD)
11.5 (24.8)
0.1 (5.8)
3.0 (4.7)
0.0 (2.9)
Median
2.4
0.0
2.0
0.0
p-value
<0.0001
<0.0001
DB RWP=Double‑blind Randomized-withdrawal Period; SD=standard deviation
14.2 Cataplexy and Excessive Daytime Sleepiness in Pediatric Narcolepsy
The effectiveness of XYWAV in pediatric patients is based upon a clinical study in patients treated with Xyrem, as described below, and additional pharmacokinetic information [see Use in Specific Populations (8.4)].
The effectiveness of Xyrem in the treatment of cataplexy and excessive daytime sleepiness in pediatric patients 7 years of age and older with narcolepsy was established in a double-blind, placebo-controlled, randomized-withdrawal study (NCT02221869). The study was conducted in 106 pediatric patients (median age: 12 years; range: 7 to 17 years) with a baseline history of at least 14 cataplexy attacks in a typical 2-week period prior to any treatment for narcolepsy symptoms. Of the 106 patients, 2 did not receive study drug and 63 patients were randomized 1:1 either to continued treatment with Xyrem or to placebo. Randomization to placebo was stopped early as the efficacy criterion was met at the pre-planned interim analysis.
Patients entered the study either taking a stable dosage of Xyrem or were Xyrem-naïve. CNS stimulants were allowed at entry, and approximately 50% of patients continued taking a stable dose of stimulant throughout the stable-dose and double-blind periods. Xyrem-naïve patients were initiated and titrated based on body weight over a period of up to 10 weeks. The total nightly dose was administered in two divided doses, with the first dose given at nighttime and the second given 2.5 to 4 hours later [see Dosage and Administration (2.2)]. Once a stable dosage of Xyrem had been achieved, these patients entered the 2-week stable-dose period; patients on a stable dosage of Xyrem at study entry remained on this dosage for 3 weeks prior to randomization. Efficacy was established at dosages ranging from 3 g to 9 g of Xyrem per night.
The primary efficacy measure was the change in frequency of cataplexy attacks. In addition, change in cataplexy severity was evaluated with the Clinical Global Impression of Change for cataplexy severity. The efficacy of Xyrem in the treatment of excessive daytime sleepiness in pediatric patients with narcolepsy was evaluated with the change in the Epworth Sleepiness Scale (Child and Adolescent) score. The Epworth Sleepiness Scale (Child and Adolescent) is a modified version of the scale used in the adult clinical trial described above. The overall change in narcolepsy condition was assessed by the Clinical Global Impression of Change for narcolepsy overall. Efficacy was assessed during or at the end of the 2-week double-blind treatment period, relative to the last 2 weeks or end of the stable-dose period (see Tables 7 and 8).
Pediatric patients taking stable dosages of Xyrem who discontinued Xyrem treatment and were randomized to placebo during the double-blind treatment period experienced a statistically significant increase in weekly cataplexy attacks compared with patients who were randomized to continue treatment with Xyrem. Patients randomized to receive placebo during the double-blind treatment period experienced a statistically significant worsening of EDS compared with patients randomized to continue receiving Xyrem (see Table 7).
Table 7: Number of Weekly Cataplexy Attacks and Epworth Sleepiness Scale (Child and Adolescent) Score Treatment Group
Baseline*,†
Double-blind Treatment Period‡,§
Median Change from Baseline
Comparison to Placebo (p-value¶)
Median Number of Cataplexy Attacks (attacks/week)
Placebo (n=32)
4.7
21.3
12.7
-
Xyrem (n=31)
3.5
3.8
0.3
<0.0001
Median Epworth Sleepiness Scale (Child and Adolescent) Score
Placebo (n=31**)
11
12
3
-
Xyrem (n=30**)
8
9
0
0.0004
* For weekly number of cataplexy attacks, baseline value is calculated from the last 14 days of the stable-dose period.
† For Epworth Sleepiness Scale score, baseline value is collected at the end of stable-dose period.
‡ Weekly number of cataplexy attacks is calculated from all days within the double-blind treatment period.
§ For Epworth Sleepiness Scale, value is collected at the end of the double-blind treatment period.
¶ P-value from rank-based analysis of covariance (ANCOVA) with treatment as a factor and rank baseline value as a covariate.
** One patient in each of the treatment groups did not have baseline ESS score available and were not included in this analysis.
Patients randomized to receive placebo during the double-blind treatment period experienced a statistically significant worsening of cataplexy severity and narcolepsy overall according to the clinician’s assessment compared with patients randomized to continue receiving Xyrem (see Table 8).
Table 8: Clinical Global Impression of Change (CGIc) for Cataplexy Severity and Narcolepsy Overall Worsened, %†
CGIc Cataplexy Severity*
CGIc Narcolepsy Overall*
Placebo
(n=32)Xyrem
(n=29)‡Placebo
(n=32)Xyrem
(n=29)‡Much worse or very much worse
66%
17%
59%
10%
p-value§
0.0001
<0.0001
* Responses indicate change of severity or symptoms relative to receiving Xyrem treatment at baseline.
† Percentages based on total number of observed values.
‡ Two patients randomized to Xyrem did not have the CGIc assessments completed and were excluded from the analysis.
§ P-value from Pearson’s chi-square test.Close14.3 Idiopathic Hypersomnia (IH) in Adults
Efficacy of XYWAV for the treatment of IH in adult patients as a once or twice nightly regimen was established in a double-blind, placebo-controlled, randomized-withdrawal, study (Study 2, NCT03533114). Study 2 consisted of a minimum of 10‑week open‑label treatment titration and optimization period (OL OTTP), (with up to 4 additional weeks) to allow for an optimally effective and tolerable dose and regimen followed by a 2‑week stable dose period (SDP), a 2‑week double-blind, randomized withdrawal period (DB RWP), and a 24‑week open label safety extension period (OLE).
Study 2 enrolled 154 patients with idiopathic hypersomnia, 19 to 75 years of age. Of the 154 patients, 115 were evaluable for efficacy data and were randomized 1:1 to continue treatment with XYWAV or to placebo in the 2‑week DB RWP. In the safety population, overall, the median age was 39 years (range: 19 to 75). At baseline, 2% of patients were taking Xyrem only, 4% of patients were taking Xyrem and an additional stimulant or alerting agent, 54% of patients were not currently taking Xyrem but were taking a stimulant or alerting agent, and 41% were treatment naïve. CNS stimulants were allowed at entry, and approximately 57% of patients continued taking a stable dose of stimulant throughout the SDP and DB RWP.
The majority of subjects were female (71%), and most were white (81%) and not Hispanic or Latino (79%).
The XYWAV dosing regimen was initiated at the discretion of the investigator according to clinical presentation. Patients were considered for XYWAV once nightly if they reported difficulty awakening as a result of sleep inertia or long sleep time. Patients were considered for twice nightly dosing if they reported disrupted nighttime sleep or difficulty with sleep maintenance. For twice nightly regimens, doses were divided equally or unequally, with the first dose administered at bedtime and the second dose administered 2.5 to 4 hours later.
Based on clinical response during the OTTP, investigators were permitted to switch patients between twice nightly and once nightly dosing regimens. When patients were switched from a twice nightly to a once nightly dosing regimen, the total nightly dose was initially the same as the first dose of the twice nightly dosing regimen. When patients were switched from a once nightly to a twice nightly dosing regimen, the total nightly dose was no more than 1.5 g higher than the current dose, divided into two doses.
At the start of the DB RWP, 23% (27/115) of patients were taking XYWAV once nightly (median nightly dose 4.5 g), and 77% (88/115) of patients were taking XYWAV twice nightly (median nightly dose 7.5 g). There were no meaningful differences in demographics, baseline characteristics or disease severity between patients receiving XYWAV once nightly vs twice nightly.
The primary efficacy endpoint was the change in Epworth Sleepiness Scale (ESS) score, as a measure of reduction in EDS from the end of the SDP to the end of the DB RWP. The ESS is an 8-item self-reported questionnaire by which patients rate their perceived likelihood of falling asleep during usual daily life activities. Each of the 8 items on the ESS is rated from 0 (would never doze) to 3 (high chance of dozing), with a maximum score of 24. Key secondary efficacy endpoints included patient global impression of change (PGIc) and the Idiopathic Hypersomnia Severity Scale (IHSS), both assessed as a change from the end of the SDP to the end of the DB RWP. The IHSS is a 14-item self-reported questionnaire assessing the severity of IH symptoms of excessive sleepiness, prolonged sleep duration, cognitive impairment, and sleep inertia. Total scores can range from 0-50, with higher scores indicating a greater severity or frequency of symptoms.
Change in ESS
Patients in Study 2 taking stable doses of XYWAV who were withdrawn from XYWAV treatment and randomized to placebo during DB RWP experienced significant worsening in ESS score compared with patients randomized to continue treatment with XYWAV (p<0.0001) across all dosing regimens (see Table 9). These two treatment groups had comparable median ESS scores (Placebo=17; XYWAV=16) at entry into the OTTP.
Table 9: Median Change in Epworth Sleepiness Scale (ESS) ESS Score
Placebo
(N=59)
XYWAV
(N=56)
Baseline End of 2-Week SDP
Median
5.0
6.5
End of 2-Week DB RWP
Median
14.0
7.0
Median Change from End of 2-Week SDP to End of 2-Week DB RWP
Median
8.0
0.0
p-value
<0.0001
SDP=Stable Dose Period
DB RWP=Double-blind Randomized-withdrawal PeriodPGIc
Patient Global Impression of change (PGIc) ratings showed that patients randomized to placebo experienced a worsening of symptoms of idiopathic hypersomnia overall compared with patients randomized to XYWAV (Table 10). The percentage of patients with worsening PGIc scores for IH overall (defined as scores of Minimally, Much Worse, or Very Much Worse) was greater for patients receiving placebo (88.1%) compared with patients receiving XYWAV (21.4%) (p<0.0001).
Table 10: PGIc* at End of the DB RWP† PGIc* IH Overall
Worsened,% ‡†
Placebo
(N=59)
n (%)XYWAV
(N=56)
n (%)Proportion Worsened (minimally, much, or very much worse)
52 (88.1)
12 (21.4)
p‑value
<0.0001
n/a
*PGIc is a 7‑point patient-reported scale by which patients rated their symptom change from “very much improved” to “very much worse.”
†DB RWP=Double‑blind Randomized-withdrawal Period.
‡At the end of the DB RWP/early termination visit, patients rated the change in their condition since the end of the Open-Label Stable-Dose Period.IHSS
At end of DB RWP, patients randomized to placebo experienced a worsening in IHSS total score, compared to patients randomized to XYWAV (p<0.0001) (see Table 11). These two treatment groups had comparable median IHSS scores (Placebo=33; XYWAV=33) at entry into the OTTP.
Table 11: Median Changes in IHSS Total Score Total Score
Placebo
(N=59)
XYWAV
(N=56)
Baseline End of 2-Week SDP
Median
14.0
14.0
End of 2-Week DB RWP
Median
29.0
16.0
Median Change from End of 2-Week SDP to End of 2-Week DB RWP
Median
14.0
0.0
p-value
<0.0001
SDP=Stable Dose Period
DB RWP=Double‑blind Randomized-withdrawal Period -
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - XYWAV is a clear to slightly opalescent oral solution. Each prescription includes at least one bottle of XYWAV with attached press in bottle adaptor, an oral measuring device ...
16.1 How Supplied
XYWAV is a clear to slightly opalescent oral solution. Each prescription includes at least one bottle of XYWAV with attached press in bottle adaptor, an oral measuring device (plastic syringe), and a Medication Guide. The pharmacy provides two empty containers with child-resistant caps with each XYWAV shipment.
Each amber bottle contains XYWAV oral solution at a concentration of 0.5 g/mL and has a child-resistant cap.
One 180 mL bottle: NDC 68727-150-01
16.2 Storage
Keep out of reach of children.
XYWAV should be stored between 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) (see USP Controlled Room Temperature).
Dispense in tight containers.
Solutions prepared following dilution should be consumed within 24 hours.
Close16.3 Handling and Disposal
XYWAV is a Schedule III drug under the Controlled Substances Act. XYWAV should be handled according to state and federal regulations. It is safe to dispose of XYWAV down the sanitary sewer.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Central Nervous System Depression - Inform patients and/or caregivers ...
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Central Nervous System Depression
Inform patients and/or caregivers that XYWAV can cause central nervous system depression, including respiratory depression, hypotension, profound sedation, syncope, and death. Instruct patients not to engage in activities requiring mental alertness or motor coordination, including operating hazardous machinery, for at least 6 hours after taking XYWAV. Instruct patients and/or their caregivers to inform their healthcare providers of all the medications they take [see Warnings and Precautions (5.1)].
Abuse and Misuse
Inform patients and/or caregivers that the active ingredient of XYWAV is gamma-hydroxybutyrate (GHB), which is associated with serious adverse reactions with illicit use and abuse [see Warnings and Precautions (5.2)].
XYWAV and XYREM REMS
XYWAV is available only through a restricted program called the XYWAV and XYREM REMS [see Warnings and Precautions (5.3)]. Inform the patient and/or caregiver of the following notable requirements:
- •
- XYWAV is dispensed only by the central pharmacy
- •
- XYWAV will be dispensed and shipped only to patients enrolled in the XYWAV and XYREM REMS
XYWAV is available only from the central pharmacy participating in the program. Therefore, provide patients and/or caregivers with the telephone number and website for information on how to obtain the product.
Alcohol or Sedative Hypnotics
Advise patients and/or caregivers that alcohol and other sedative hypnotics should not be taken with XYWAV [see Contraindications (4)].
Sedation
Inform patients and/or caregivers that the patient is likely to fall asleep quickly after taking XYWAV (often within 5 and usually within 15 minutes), but the time it takes to fall asleep can vary from night to night. The sudden onset of sleep, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization [see Adverse Reactions (6.2)]. Instruct patients and/or caregivers that the patient should remain in bed following ingestion of each dose. Instruct patients and/or caregivers that the patient should not take a subsequent nightly dose until at least 2.5 to 4 hours after the previous dose [see Dosage and Administration (2.4)].
Administration Instructions
Inform patients to administer XYWAV at least 2 hours after eating. Inform patients and/or caregivers of patients taking XYWAV twice nightly, that the total nightly dosage of XYWAV is divided into two doses [see Dosage and Administration (2.4)].
Inform patients if their nightly dose requires multiple draws. Instruct patients on how to perform the draws from the bottle.
Respiratory Depression and Sleep-Disordered Breathing
Inform patients that XYWAV may impair respiratory drive, especially in patients with compromised respiratory function, and may cause apnea [see Warnings and Precautions (5.4)].
Depression and Suicidality
Instruct patients and/or caregivers to contact a healthcare provider immediately if the patient develops depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or suicidal ideation [see Warnings and Precautions (5.5)].
Other Behavioral or Psychiatric Adverse Reactions
Inform patients and/or caregivers that XYWAV can cause behavioral or psychiatric adverse reactions, including confusion, anxiety, and psychosis. Instruct them to notify their healthcare provider if any of these types of symptoms occur [see Warnings and Precautions (5.6)].
Sleepwalking
Instruct patients and/or caregivers that XYWAV has been associated with sleepwalking and other behaviors during sleep, and to contact their healthcare provider if this occurs [see Warnings and Precautions (5.7)].
Close
Distributed By:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94304
Protected by U.S. Patent Nos. 8,591,922; 8,772,306; 8,901,173; 9,050,302; 9,132,107; 9,486,426; 9,555,017; 10,195,168; 10,213,400; 10,675,258; 10,864,181; 11,253,494; 11,554,102; and 11,426,373. -
MEDICATION GUIDEMEDICATION GUIDE - XYWAV® (ZYE wave) (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII - Read this Medication Guide carefully before you start or your child starts ...
MEDICATION GUIDE
XYWAV® (ZYE wave)
(calcium, magnesium, potassium, and sodium oxybates)
oral solution, CIIIRead this Medication Guide carefully before you start or your child starts taking XYWAV, and each time you get or your child gets a refill. There may be new information. This information does not take the place of talking to your doctor about your or your child’s medical condition or treatment.
What is the most important information I should know about XYWAV?
- •
- XYWAV is a central nervous system (CNS) depressant. Taking XYWAV with other CNS depressants, such as medicines used to make you or your child fall asleep, including opioid analgesics, benzodiazepines, sedating antidepressants, antipsychotics, sedating anti-epileptic medicines, general anesthetics, muscle relaxants, alcohol, or street drugs, may cause serious medical problems, including:
- o
- trouble breathing (respiratory depression)
- o
- low blood pressure (hypotension)
- o
- changes in alertness (drowsiness)
- o
- fainting (syncope)
- o
- death
Ask your doctor if you are not sure if you are, or your child is, taking a medicine listed above.
- •
- XYWAV is a federal controlled substance (CIII). The active ingredient of XYWAV is a form of gamma-hydroxybutyrate (GHB) that is also a federal controlled substance (CI). Abuse of illegal GHB, either alone or with other CNS depressants, may cause serious medical problems, including:
- o
- seizure
- o
- trouble breathing (respiratory depression)
- o
- changes in alertness (drowsiness)
- o
- coma
- o
- death
Call your doctor right away if you have or your child has any of these serious side effects.
- •
- Anyone who takes XYWAV should not do anything that requires them to be fully awake or is dangerous, including driving a car, using heavy machinery, or flying an airplane, for at least 6 hours after taking XYWAV. Those activities should not be done until you know how XYWAV affects you or your child.
- •
- Keep XYWAV in a safe place to prevent abuse and misuse. Selling or giving away XYWAV may harm others and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- •
- Because of the risk of CNS depression, abuse, and misuse, XYWAV is available only by prescription, and filled through the central pharmacy in the XYWAV and XYREM REMS. You or your child must be enrolled in the XYWAV and XYREM REMS to receive XYWAV. For information on how to receive XYWAV, visit www.XYWAVXYREMREMS.com. Before you receive or your child receives XYWAV, your doctor or pharmacist will make sure that you understand how to take XYWAV safely and effectively. If you have any questions about XYWAV, ask your doctor or call the XYWAV and XYREM REMS at 1-866-997-3688.
What is XYWAV?
XYWAV is a prescription medicine used to treat:
- •
- the following symptoms in people 7 years of age or older with narcolepsy:
- o
- sudden onset of weak or paralyzed muscles (cataplexy), or
- o
- excessive daytime sleepiness (EDS)
- •
- idiopathic hypersomnia (IH) in adults
It is not known if XYWAV is safe and effective in children less than 7 years of age with narcolepsy.
It is not known if XYWAV is safe and effective in children with IH.
Do not take XYWAV if you or your child:
- •
- takes other sleep medicines or sedatives (medicines that cause sleepiness)
- •
- drinks alcohol
- •
- has a rare problem called succinic semialdehyde dehydrogenase deficiency
Before taking XYWAV, tell your doctor about all medical conditions, including if you or your child:
- •
- have a history of drug abuse.
- •
- have short periods of not breathing while sleeping (sleep apnea).
- •
- has trouble breathing or has lung problems. You or your child may have a higher chance of having serious breathing problems when taking XYWAV.
- •
- have or had depression or has tried to harm yourself or themselves. You or your child should be watched carefully for new symptoms of depression.
- •
- has or had behavior or other psychiatric problems such as:
- o
- anxiety
- o
- feeling more suspicious (paranoia)
- o
- acting aggressive
- o
- seeing or hearing things that are not real (hallucinations)
- o
- being out of touch with reality (psychosis)
- o
- agitation
- •
- have liver problems.
- •
- are pregnant or plan to become pregnant. It is not known if XYWAV can harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. XYWAV passes into breast milk. You and your doctor should decide if you or your child will take XYWAV or breastfeed.
Tell your doctor about all the medicines you take or your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially, tell your doctor if you take or your child takes other medicines to help you or your child sleep (sedatives). Know the medicines you take or your child takes. Keep a list of them to show your doctor and pharmacist when you get or your child gets a new medicine.
How should I take or give XYWAV?
- •
- Read the Instructions for Use at the end of this Medication Guide for detailed instructions on how to take XYWAV.
- •
- Take or give XYWAV exactly as your doctor tells you to take or give it. Your doctor may change the dose or dosing routine if needed.
- •
- Wait at least 2 hours after eating before taking or giving XYWAV.
- •
- XYWAV can cause physical dependence and craving for the medicine when it is not taken as directed.
- •
- Never change the dose without talking to your doctor.
- •
- XYWAV can cause sleep very quickly without feeling drowsy. Some people fall asleep within 5 minutes and most fall asleep within 15 minutes. The time it takes to fall asleep might be different from night to night.
- •
- Falling asleep quickly, including while standing or while getting up from the bed, has led to falls with injuries that have required some people to be hospitalized.
- •
- XYWAV can be taken 1 time or 2 times a night as prescribed by your doctor.
- •
-
If you or your child have been prescribed XYWAV 2 times a night: divide the total nightly dose into 2 doses to be taken at bedtime and 2½ to 4 hours later.
- o
- Adults: Take the first XYWAV dose at bedtime while you are in bed and lie down immediately. Take the second XYWAV dose 2½ to 4 hours after the first XYWAV dose. You may want to set an alarm clock to make sure you wake up to take the second XYWAV dose. You should remain in bed after taking the first and second doses of XYWAV.
- o
- Children: Give the first XYWAV dose at bedtime or after an initial period of sleep, while your child is in bed and have them lie down immediately. Give the second XYWAV dose 2½ to 4 hours after the first XYWAV dose. You may want to set an alarm clock to make sure you wake up to give the second XYWAV dose. Your child should remain in bed after taking the first and second doses of XYWAV.
- o
- If you miss or your child misses the second XYWAV dose, skip that dose and do not take or give XYWAV again until the next night. Never take or give 2 XYWAV doses at 1 time.
- •
- If you have been prescribed XYWAV 1 time a night: Take your XYWAV dose at bedtime while you are in bed and lie down immediately. You should remain in bed after taking XYWAV.
- •
- If you take or your child takes too much XYWAV, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of XYWAV?
XYWAV can cause serious side effects, including:
- •
- See “What is the most important information I should know about XYWAV?”
- •
-
breathing problems, including:
- o
- slower breathing.
- o
- trouble breathing.
- o
- short periods of not breathing while sleeping (sleep apnea). People who already have breathing or lung problems have a higher chance of having breathing problems when they take XYWAV.
- •
-
mental health problems, including:
- o
- confusion
- o
- seeing or hearing things that are not real (hallucinations)
- o
- unusual or disturbing thoughts (abnormal thinking)
- o
- feeling anxious or upset
- o
- depression
- o
- thoughts of killing yourself or trying to kill yourself
- o
- increased tiredness
- o
- feelings of guilt or worthlessness
- o
- difficulty concentrating
Call your doctor right away if you have or your child has symptoms of mental health problems, or a change in weight or appetite.
- •
- sleepwalking. Sleepwalking can cause injuries. Call your doctor if you start or your child starts sleepwalking. Your doctor should check you or your child.
The most common side effects of XYWAV in adults with narcolepsy or IH include:
- •
- nausea
- •
- headache
- •
- dizziness
- •
- anxiety
- •
- insomnia
- •
- decreased appetite
- •
- excessive sweating (hyperhidrosis)
- •
- vomiting
- •
- diarrhea
- •
- dry mouth
- •
- parasomnia (a sleep disorder that can include abnormal dreams, abnormal rapid eye movement (REM) sleep, sleep paralysis, sleep talking, sleep terror, sleep-related eating disorder, sleepwalking and other abnormal sleep-related events)
- •
- somnolence
- •
- fatigue
- •
- tremor
The most common side effects of XYREM (which also contains oxybate like XYWAV) in children include:
- •
- nausea
- •
- bedwetting
- •
- vomiting
- •
- headache
- •
- weight decrease
- •
- decreased appetite
- •
- dizziness
- •
- sleepwalking
These are not all the possible side effects of XYWAV. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store XYWAV?
- •
- Store XYWAV in the original bottle prior to mixing with water. After mixing with water, store XYWAV in the pharmacy containers with child-resistant caps provided by the pharmacy.
- •
- Store XYWAV at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- XYWAV solution prepared after mixing with water should be taken within 24 hours.
- •
- When you have finished using a XYWAV bottle:
- o
- empty any unused XYWAV down the sink drain.
- o
- cross out the label on the XYWAV bottle with a marker.
- o
- place the empty XYWAV bottle in the trash.
XYWAV comes in a child-resistant package.
Keep XYWAV and all medicines out of the reach of children and pets.
General information about the safe and effective use of XYWAV.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XYWAV for a condition for which it was not prescribed. Do not give XYWAV to other people, even if they have the same symptoms. It may harm them.
You can ask your pharmacist or doctor for information about XYWAV that is written for health professionals.
What are the ingredients in XYWAV?
Active ingredients: calcium oxybate, magnesium oxybate, potassium oxybate, and sodium oxybate (gamma-hydroxybutyrate (GHB)).Inactive ingredients: purified water and sucralose
Distributed By:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94304
For more information, go to www.XYWAVXYREMREMS.com or call the XYWAV and XYREM REMS at 1-866-997-3688.
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 08/2021
CloseINSTRUCTIONS FOR USE
XYWAV® (ZYE wave)
(calcium, magnesium, potassium, and sodium oxybates)
oral solution, CIIIThis Instructions for Use contains information on how to take XYWAV. Read this Instructions for Use carefully before you (or your child) start taking XYWAV and each time you (or your child) get a refill. There may be new information. This information does not take the place of talking to your doctor about your (or your child’s) medical condition or treatment.
Important Information:
- •
-
Your doctor and pharmacist will provide instructions to take XYWAV 2 times a night or 1 time a night.
- o
- For 2 times a night, you will need to split your (or your child’s) prescribed XYWAV dose into 2 separate pharmacy containers for mixing.
- o
- For 1 time a night, you will use the dosing syringe to draw up your prescribed XYWAV dose and empty the dose into 1 pharmacy container for mixing. You will only need 1 pharmacy container to prepare your dose.
- •
- You (or your child) should take XYWAV while in bed. Lie down immediately after taking XYWAV and remain in bed afterwards.
- •
- You will need to mix XYWAV with water before you take or give your child the dose.
- •
- Safely store the prepared XYWAV doses and take within 24 hours after mixing. If the prepared dose was not taken within this time, throw the mixture away. See “Throwing away (disposing of) XYWAV” section below for instructions about how to safely throw away XYWAV.
- •
- The pharmacy container(s) may be rinsed out with water and emptied into the sink drain.
Supplies you will need for mixing and taking (or giving your child) XYWAV. See Figure A:
- •
- Bottle of XYWAV medicine
- •
- Dosing syringe for measuring and dispensing the XYWAV dose
- •
- Measuring cup that is able to measure about ¼ cup of water (not provided with the XYWAV shipment)
- •
- 1 or 2 empty pharmacy containers with child-resistant caps for mixing, storing, and taking the XYWAV doses
- •
- Alarm clock if you take XYWAV 2 times a night (not pictured)
- •
- Medication Guide
Note: If you or your child are prescribed XYWAV 2 times a night, see the instructions for 2 times a night dosing. If you are prescribed XYWAV 1 time a night, see the instructions for 1 time a night dosing.
How should I store XYWAV?
- •
- Store XYWAV in the original bottle prior to mixing with water. After mixing, store XYWAV in the pharmacy containers provided by the pharmacy. The cap on the original bottle is child-resistant. The pharmacy container cap is child‑resistant only when the child-resistant side of the cap is used.
- •
- Store XYWAV at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- XYWAV solution prepared after mixing with water should be taken within 24 hours or emptied down the sink drain.
Throwing away (disposing of) XYWAV
- •
- When you have finished using a XYWAV bottle:
- o
- empty any unused XYWAV down the sink drain
- o
- cross out the label on the XYWAV bottle with a marker (not provided with the XYWAV shipment)
- o
- place the empty XYWAV bottle in the trash
- •
- Keep XYWAV and all medicines out of the reach of children and pets.
Distributed By:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94304
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 4/2023 -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information
XYWAV calcium, magnesium, potassium, and sodium oxybates solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68727-150 Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM OXYBATE (UNII: 7G33012534) (OXYBATE - UNII:30IW36W5B2) SODIUM OXYBATE 0.5 g in 1 mL Calcium Oxybate (UNII: 8W24SYD6ZI) (OXYBATE - UNII:30IW36W5B2) Calcium Oxybate 0.5 g in 1 mL Potassium Oxybate (UNII: S8NKF3H3KT) (OXYBATE - UNII:30IW36W5B2) Potassium Oxybate 0.5 g in 1 mL Magnesium Oxybate (UNII: G983HLV265) (OXYBATE - UNII:30IW36W5B2) Magnesium Oxybate 0.5 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68727-150-01 180 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212690 11/02/2020 Labeler - Jazz Pharmaceuticals, Inc. (135926363) Registrant - Jazz Pharmaceuticals, Inc. (135926363)
CloseEstablishment Name Address ID/FEI Business Operations Jazz Pharmaceuticals Ireland Limited 986019606 MANUFACTURE(68727-150) , ANALYSIS(68727-150) , PACK(68727-150)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
XYWAV- calcium, magnesium, potassium, and sodium oxybates solution
Number of versions: 12
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Oct 10, 2024 | 14 (current) | download |
| Aug 19, 2024 | 13 | download |
| Apr 28, 2023 | 12 | download |
| Apr 17, 2023 | 11 | download |
| Oct 7, 2022 | 10 | download |
| Mar 11, 2022 | 9 | download |
| Aug 24, 2021 | 8 | download |
| Jul 7, 2021 | 7 | download |
| Feb 24, 2021 | 6 | download |
| Nov 13, 2020 | 5 | download |
| Oct 20, 2020 | 4 | download |
| Aug 5, 2020 | 3 | download |
RxNorm
XYWAV- calcium, magnesium, potassium, and sodium oxybates solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2393794 | xywav 0.5 GM in 1 mL Oral Solution | PSN |
| 2 | 2393794 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / potassium oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution [Xywav] | SBD |
| 3 | 2393794 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / K+ oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution [Xywav] | SY |
| 4 | 2393794 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / Pot oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution [Xywav] | SY |
| 5 | 2393794 | Xywav 0.5 GM per 1 ML Oral Solution | SY |
| 6 | 2393794 | Xywav (calcium oxybate 0.234 GM / magnesium oxybate 0.096 GM / potassium oxybate 0.13 GM / sodium oxybate 0.04 GM) per 1 ML Oral Solution | SY |
| 7 | 2393795 | calcium / magnesium / potassium / sodium oxybates 0.5 GM in 1 mL Oral Solution | PSN |
| 8 | 2393795 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / potassium oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution | SCD |
| 9 | 2393795 | calcium/magnesium/potassium/sodium oxybates 0.5 GM per 1 ML Oral Solution | SY |
| 10 | 2393795 | calcium oxybate 0.234 GM / magnesium oxybate 0.096 GM / potassium oxybate 0.13 GM / sodium oxybate 0.04 GM per 1 ML Oral Solution | SY |
| 11 | 2393795 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / K+ oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution | SY |
| 12 | 2393795 | calcium oxybate 234 MG/ML / magnesium oxybate 96 MG/ML / Pot oxybate 130 MG/ML / sodium oxybate 40 MG/ML Oral Solution | SY |
Get Label RSS Feed for this Drug
XYWAV- calcium, magnesium, potassium, and sodium oxybates solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=1e0ae43a-037f-42af-8e23-a0e51d75abe8
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
XYWAV- calcium, magnesium, potassium, and sodium oxybates solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 68727-150-01 |