Label: VYXEOS (- daunorubicin and cytarabine liposome injection, powder, lyophilized, for suspension
- NDC Code(s): 68727-745-01, 68727-745-02, 68727-745-05
- Packager: Jazz Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VYXEOS safely and effectively. See full prescribing information for VYXEOS.
VYXEOS® (daunorubicin and cytarabine) liposome for injection, for intravenous use
Initial U.S. Approval: 2017WARNING: DO NOT INTERCHANGE WITH OTHER DAUNORUBICIN AND/OR CYTARABINE-CONTAINING PRODUCTS
See full prescribing information for complete boxed warning.
- •
- VYXEOS has different dosage recommendations than daunorubicin hydrochloride injection, cytarabine injection, daunorubicin citrate liposome injection, and cytarabine liposome injection. Verify drug name and dose prior to preparation and administration to avoid dosing errors (5.1).
INDICATIONS AND USAGE
VYXEOS is a liposomal combination of daunorubicin, an anthracycline topoisomerase inhibitor, and cytarabine, a nucleoside metabolic inhibitor, that is indicated for the treatment of newly-diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in adults and pediatric patients 1 year and older. (1)
DOSAGE AND ADMINISTRATION
- •
- Induction: VYXEOS (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome via intravenous infusion over 90 minutes on days 1, 3, and 5 and on days 1 and 3 for subsequent cycles of induction, if needed. (2.1)
- •
- Consolidation: VYXEOS (daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) liposome via intravenous infusion over 90 minutes on days 1 and 3. (2.1)
- •
- See Full Prescribing Information for instructions on preparation and administration. (2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 44 mg daunorubicin and 100 mg cytarabine encapsulated in liposomes as a lyophilized cake in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
- •
- History of serious hypersensitivity to daunorubicin, cytarabine or any components of the formulation. (4)
WARNINGS AND PRECAUTIONS
- •
- Hemorrhage: Serious or fatal hemorrhagic events with associated prolonged thrombocytopenia have occurred with VYXEOS. Monitor blood counts regularly until recovery. (5.2)
- •
- Cardiotoxicity: VYXEOS treatment is not recommended in patients with cardiac function that is less than normal. Discontinue VYXEOS in patients with impaired cardiac function unless the benefit of continuing treatment outweighs the risk. (2.2, 5.3)
- •
- Hypersensitivity: If severe or life-threatening hypersensitivity reaction occurs, discontinue VYXEOS, treat according to standard of care, and monitor until signs and symptoms resolve. (2.2, 5.4)
- •
- Tissue Necrosis: Daunorubicin has been associated with local tissue necrosis at the site of drug extravasation. Confirm intravenous access before administration. (5.6)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.7, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 25%) are hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals, Inc. at 1-800-520-5568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DO NOT INTERCHANGE WITH OTHER DAUNORUBICIN AND/OR CYTARABINE-CONTAINING PRODUCTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modification
2.3 Preparation and Handling Instructions
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Do Not Interchange With Other Daunorubicin And/Or Cytarabine-Containing Products
5.2 Hemorrhage
5.3 Cardiotoxicity
5.4 Hypersensitivity Reactions
5.5 Copper Overload
5.6 Tissue Necrosis
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cardiotoxic Agents
7.2 Hepatotoxic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DO NOT INTERCHANGE WITH OTHER DAUNORUBICIN AND/OR CYTARABINE-CONTAINING PRODUCTS
- •
- VYXEOS has different dosage recommendations than daunorubicin hydrochloride injection, cytarabine injection, daunorubicin citrate liposome injection, and cytarabine liposome injection. Verify drug name and dose prior to preparation and administration to avoid dosing errors [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
A full VYXEOS course consists of 1-2 cycles of Induction and up to 2 cycles of Consolidation at the dose and schedule listed in Table 1. Prior to initiating each cycle of VYXEOS, calculate the prior cumulative anthracycline exposure for the patient [see Warnings and Precautions (5.3)]. Administer prophylactic anti-emetics before treatment with VYXEOS.
Table 1: Dose and Schedule for VYXEOS Cycle
VYXEOS Dose and Schedule
First Induction
(daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome days 1, 3, and 5
Second Induction a
(daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome days 1 and 3
Consolidation
(daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) liposome days 1 and 3
a Only for patients failing to achieve a response with the first induction cycle.
For the first cycle of induction, the recommended dose of VYXEOS is (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome administered via intravenous infusion over 90 minutes on days 1, 3, and 5. Prior to initiating induction, assess cardiac function and obtain liver and renal function studies. For patients who do not achieve remission with the first induction cycle, a second induction cycle may be administered 2 to 5 weeks after the first if there was no unacceptable toxicity with VYXEOS. The recommended dose for the second induction cycle of VYXEOS is (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome administered via intravenous infusion over 90 minutes on days 1 and 3.
Administer the first consolidation cycle 5 to 8 weeks after the start of the last induction. The recommended dose for each cycle of consolidation therapy is VYXEOS (daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) liposome administered via intravenous infusion over 90 minutes on days 1 and 3.
Assess cardiac function, complete blood counts, liver and renal function before each consolidation cycle. Do not start VYXEOS consolidation until the absolute neutrophil count recovers to greater than 0.5 Gi/L and the platelet count recovers to greater than 50 Gi/L in the absence of unacceptable toxicity. Administer the second consolidation cycle 5 to 8 weeks after the start of the first consolidation cycle in patients who do not show disease progression or unacceptable toxicity to VYXEOS.
2.2 Dosage Modification
Missed Doses of VYXEOS
If a planned dose of VYXEOS is missed, administer the dose as soon as possible and adjust the dosing schedule accordingly, maintaining the treatment interval.
Hypersensitivity Reactions
For hypersensitivity reactions of any grade/severity, interrupt VYXEOS infusion immediately and manage symptoms. Reduce the rate of infusion or discontinue treatment as outlined below [see Warnings and Precautions (5.4)].
- •
- Mild symptoms: Once symptoms resolve, reinitiate infusion at half the prior rate of infusion. Consider premedication with antihistamines and/or corticosteroids for subsequent doses of VYXEOS.
- •
- Moderate symptoms: Do not reinitiate infusion. For subsequent doses of VYXEOS, premedicate with antihistamines and/or corticosteroids prior to initiating infusion at same rate.
- •
- Severe/life-threatening symptoms: Permanently discontinue VYXEOS treatment, treat according to the standard of care to manage symptoms, and monitor patient until symptoms resolve.
Cardiotoxicity
Discontinue VYXEOS in patients who exhibit impaired cardiac function unless the benefit of continuing treatment outweighs the risk [see Warnings and Precautions (5.3)].
2.3 Preparation and Handling Instructions
VYXEOS is a hazardous drug. Follow applicable special handling and disposal procedures.1 VYXEOS is supplied as a single-dose vial and does not contain any preservatives. Do not save any unused portions for later administration.
Reconstitute and further dilute VYXEOS prior to intravenous infusion.
Reconstitution:
- •
- Calculate the VYXEOS dose based on daunorubicin and individual patient's BSA.
- •
- Calculate the number of vials of VYXEOS based on the daunorubicin dose.
- •
- Remove the appropriate number of vials of VYXEOS from the refrigerator and equilibrate to the room temperature for 30 minutes.
- •
- Then, reconstitute each vial with 19 mL of Sterile Water for Injection using a sterile syringe and immediately thereafter start a 5-minute timer.
- •
- Carefully swirl the contents of the vial for 5 minutes while gently inverting the vial every 30 seconds.
- •
- Do not heat, vortex, or shake vigorously.
- •
- After reconstitution, let rest for 15 minutes.
- •
- The reconstituted product should be an opaque, purple, homogeneous dispersion, essentially free from visible particulates. After reconstitution (but before final dilution), each mL will contain 2.2 mg of daunorubicin and 5 mg of cytarabine.
- •
- Use the reconstituted solution immediately. If needed, store the reconstituted solution in the vial refrigerated at 2ºC to 8ºC (36°F to 46°F) for up to 4 hours. Note that the reconstituted product in the vial and the reconstituted product which has been diluted into an infusion solution are stable for a total of 4 hours (not 4 hours each) when stored at 2°C to 8°C.
Dilution:
- •
- Calculate the volume of reconstituted VYXEOS required using the following formula:
[volume required (mL) = dose of daunorubicin (mg/m2) X patient's BSA (m2) ÷ 2.2 (mg/mL)] - •
- Gently invert each vial 5 times prior to withdrawing the reconstituted product for further dilution.
- •
- Aseptically withdraw the calculated volume of the reconstituted product from the vial(s) with a sterile syringe and transfer it to an infusion bag containing 500 mL of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. There may be residual product remaining in the vial. Discard unused portion.
- •
- Gently invert the bag to mix the solution. The dilution of the reconstituted product results in a deep purple, translucent, homogeneous dispersion, free from visible particulates.
- •
- If the diluted infusion solution is not used immediately, store in a refrigerator at 2ºC to 8ºC (36°F to 46°F) for up to 4 hours. If the reconstituted solution in the vial was stored for 4 hours, the diluted infusion solution must be used immediately and cannot be stored for an additional 4 hours.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Only solutions without visible particles should be used.
2.4 Administration Instructions
- •
- For intravenous use only.
- •
- Do not mix VYXEOS with or administer as an infusion with other drugs.
- •
- Administer VYXEOS by constant intravenous infusion over 90 minutes via an infusion pump through a central venous catheter or a peripherally inserted central catheter. An in-line membrane filter may be used for the intravenous infusion of Vyxeos, provided the minimum pore diameter of the filter is greater than or equal to 15 µm.
- •
- Flush the line after administration with 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
The use of VYXEOS is contraindicated in patients with the following:
- •
- History of serious hypersensitivity reaction to cytarabine, daunorubicin, or any component of the formulation [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Do Not Interchange With Other Daunorubicin And/Or Cytarabine-Containing Products
Due to substantial differences in the pharmacokinetic parameters, the dose and schedule recommendations for VYXEOS are different from those for daunorubicin hydrochloride injection, cytarabine injection, daunorubicin citrate liposome injection, and cytarabine liposome injection. Verify drug name and dose prior to preparation and administration to avoid dosing errors. Do not substitute other preparations of daunorubicin or cytarabine for VYXEOS.
5.2 Hemorrhage
Serious or fatal hemorrhage events, including fatal central nervous system (CNS) hemorrhages, associated with prolonged severe thrombocytopenia, have occurred in patients treated with VYXEOS. In Study 1 (NCT01696084), the incidence of any grade hemorrhagic events during the entire treatment period was 74% of patients on the VYXEOS arm and 56% on the control arm. The most frequently reported hemorrhagic event was epistaxis (36% in VYXEOS arm and 18% in control arm). Grade 3 or greater events occurred in 12% of VYXEOS treated patients and 8% of patients treated with 7+3. Fatal treatment-emergent CNS hemorrhage not in the setting of progressive disease occurred in 2% of patients on the VYXEOS arm and in 0.7% of patients on the control arm. Monitor blood counts regularly until recovery and administer platelet transfusion support as required [see Adverse Reactions (6.1)].
5.3 Cardiotoxicity
VYXEOS contains the anthracycline daunorubicin, which has a known risk of cardiotoxicity. Prior therapy with anthracyclines, pre-existing cardiac disease, previous radiotherapy to the mediastinum, or concomitant use of cardiotoxic drugs may increase the risk of daunorubicin-induced cardiac toxicity. Prior to administering VYXEOS, obtain an electrocardiogram (ECG) and assess cardiac function by multi-gated radionuclide angiography (MUGA) scan or echocardiography (ECHO). Repeat MUGA or ECHO determinations of left ventricular ejection fraction (LVEF) prior to consolidation with VYXEOS and as clinically required. Discontinue VYXEOS in patients with impaired cardiac function unless the benefit of initiating or continuing treatment outweighs the risk. VYXEOS treatment is not recommended in patients with LVEF that is less than normal.
Total cumulative doses of non-liposomal daunorubicin greater than 550 mg/m2 have been associated with an increased incidence of drug-induced congestive heart failure. The tolerable limit appears lower (400 mg/m2) in patients who received radiation therapy to the mediastinum.
Calculate the lifetime cumulative anthracycline exposure prior to each cycle of VYXEOS. VYXEOS treatment is not recommended in patients whose lifetime anthracycline exposure has reached the maximum cumulative limit. The exposure to daunorubicin following each cycle of VYXEOS is shown in Table 2.
Table 2: Cumulative Exposure of Daunorubicin per Cycle of VYXEOS Therapy
Daunorubicin per Dose
Number of Doses per Cycle
Daunorubicin per Cycle
First Induction Cycle
44 mg/m2
3
132 mg/m2
Second Induction Cycle
44 mg/m2
2
88 mg/m2
Each Consolidation Cycle
29 mg/m2
2
58 mg/m2
5.4 Hypersensitivity Reactions
Serious or fatal hypersensitivity reactions, including anaphylactic reactions, have been reported with daunorubicin and cytarabine. Monitor patients for hypersensitivity reactions. If a mild or moderate hypersensitivity reaction occurs, interrupt or slow the rate of infusion with VYXEOS and manage symptoms. If a severe or life-threatening hypersensitivity reaction occurs, discontinue VYXEOS permanently, treat symptoms according to the standard of care, and monitor until symptoms resolve [see Dosage and Administration (2.2)].
5.5 Copper Overload
Reconstituted VYXEOS contains 5 mg/mL copper gluconate, of which 14% is elemental copper. There is no clinical experience with VYXEOS in patients with Wilson’s disease or other copper-related metabolic disorders. The maximum theoretical total exposure of copper under the recommended VYXEOS dosing regimen is 106 mg/m2[see Dosage and Administration (2.1)]. Consult with a hepatologist and nephrologist with expertise in managing acute copper toxicity in patients with Wilson’s disease treated with VYXEOS. Monitor total serum copper, serum non-ceruloplasmin bound copper, 24-hour urine copper levels and serial neuropsychological examinations in these patients. Use VYXEOS in patients with Wilson’s disease only if the benefits outweigh the risks. Discontinue VYXEOS in patients with signs or symptoms of acute copper toxicity.
5.6 Tissue Necrosis
Daunorubicin has been associated with severe local tissue necrosis at the site of drug extravasation. Administer VYXEOS by the intravenous route only. Confirm patency of the intravenous access before administration. Do not administer by the intramuscular or subcutaneous route.
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies with daunorubicin and cytarabine, VYXEOS can cause embryo-fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of VYXEOS, daunorubicin, or cytarabine in pregnant women. Daunorubicin and cytarabine are reproductive and developmental toxicants in multiple species (mice, rats, and/or dogs), starting at a dose that was approximately 0.02 times the exposure in patients at the recommended human dose on an mg/m2 basis. Patients should be advised to avoid becoming pregnant while taking VYXEOS. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, apprise the patient of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of VYXEOS [see Use in Specific Populations (8.1) and (8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Hemorrhage [see Warnings and Precautions (5.2)]
- •
- Cardiotoxicity [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- •
- Copper Overload [see Warnings and Precautions (5.5)]
- •
- Tissue Necrosis [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VYXEOS was determined in a randomized trial for adults with newly-diagnosed t‑AML or AML-MRC [see Clinical Studies (14)] which included 153 patients treated with VYXEOS and 151 patients treated with a standard combination of cytarabine and daunorubicin (7+3). At study entry, patients were required to have a LVEF of at least 50% and a prior lifetime cumulative anthracycline exposure less than 368 mg/m2 daunorubicin (or equivalent). On study, the median number of cycles administered was 2 (range, 1–4 cycles) on the VYXEOS arm and 1 (range, 1–4 cycles) on the control arm. The median cumulative daunorubicin dose was 189 mg/m2 (range, 44–337 mg/m2) on the VYXEOS arm and 186 mg/m2 (range, 44–532 mg/m2) on the control arm.
Nine patients each on the VYXEOS arm (6%) and the control arm (6%) had a fatal adverse reaction on treatment or within 30 days of therapy that was not in the setting of progressive disease. Fatal adverse reactions on the VYXEOS arm included infection, CNS hemorrhage, and respiratory failure. Overall, all-cause day-30 mortality was 6% in the VYXEOS arm and 11% in the control arm. During the first 60 days of the study, 14% (21/153) of patients died in the VYXEOS arm vs. 21% (32/151) of patients in the 7+3 treatment group.
The most common serious adverse reactions (incidence ≥ 5%) on the VYXEOS arm were dyspnea, myocardial toxicity, sepsis, pneumonia, febrile neutropenia, bacteremia and hemorrhage. Adverse reactions led to discontinuation of VYXEOS in 18% (28/153) of patients, and 13% (20/151) in the control arm. The adverse reactions leading to discontinuation on the VYXEOS arm included prolonged cytopenias, infection, cardiotoxicity, respiratory failure, hemorrhage (GI and CNS), renal insufficiency, colitis, and generalized medical deterioration. The most common adverse reactions (incidence ≥ 25%) in patients on the VYXEOS arm were hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting. The incidences of common adverse drug reactions during the induction phase in Study 1 are presented in Table 3.
Table 3: Common Adverse Reactions (≥ 10% Incidence in the VYXEOS arm) During the Induction Phase Adverse Reaction a
All Grades b
Grades 3 to 5 b
VYXEOS
N=153
n (%)
7+3
N=151
n (%)
VYXEOS
N=153
n (%)
7+3
N=151
n (%)
Hemorrhage a
107 (70)
74 (49)
15 (10)
9 (6)
Febrile Neutropenia
104 (68)
103 (68)
101 (66)
102 (68)
Rash a
82 (54)
55 (36)
8 (5)
2 (1)
Edema a
78 (51)
90 (60)
2 (2)
5 (3)
Nausea
72 (47)
79 (52)
1 (1)
1 (1)
Diarrhea/Colitis a
69 (45)
100 (66)
4 (3)
10 (7)
Mucositis a
67 (44)
69 (46)
2 (1)
7 (5)
Constipation
61 (40)
57 (38)
0
0
Musculoskeletal pain a
58 (38)
52 (34)
5 (3)
4 (3)
Abdominal pain a
51 (33)
45 (30)
3 (2)
3 (2)
Cough a
51 (33)
34 (23)
0
1 (1)
Headache a
51 (33)
36 (24)
2 (1)
1 (1)
Dyspnea a
49 (32)
51 (34)
17 (11)
15 (10)
Fatigue a
49 (32)
58 (38)
8 (5)
8 (5)
Arrhythmia a
46 (30)
41 (27)
10 (7)
7 (5)
Decreased appetite
44 (29)
57 (38)
2 (1)
5 (3)
Pneumonia (excluding fungal) a
39 (26)
35 (23)
30 (20)
26 (17)
Sleep disorders a
38 (25)
42 (28)
2 (1)
1 (1)
Bacteremia (excluding sepsis) a
37 (24)
37 (25)
35 (23)
31 (21)
Vomiting a
37 (24)
33 (22)
0
0
Chills
35 (23)
38 (25)
0
0
Hypotension a
30 (20)
32 (21)
7 (5)
1 (1)
Non-conduction cardiotoxicity a
31 (20)
27 (18)
13 (9)
15 (10)
Dizziness a
27 (18)
26 (17)
1 (1)
0
Fungal infection a
27 (18)
19 (13)
11 (7)
9 (6)
Hypertension a
28 (18)
22 (15)
15 (10)
8 (5)
Hypoxia a
28 (18)
31 (21)
19 (12)
23 (15)
Upper respiratory infections (excluding fungal) a
28 (18)
19 (13)
4 (3)
1 (1)
Chest pain a
26 (17)
22 (15)
5 (3)
0
Pyrexia
26 (17)
23 (15)
1 (1)
2 (1)
Catheter/device/injection site reaction a
24 (16)
15 (10)
0
0
Delirium a
24 (16)
33 (22)
4 (3)
9 (6)
Pleural effusion
24 (16)
25 (17)
3 (2)
2 (1)
Anxiety
21 (14)
16 (11)
0
0
Pruritus a
23 (15)
14 (9)
0
0
Sepsis (excluding fungal) a
17 (11)
20 (13)
n/a
Hemorrhoids
16 (11)
12 (8)
0
0
Petechiae
17 (11)
17 (11)
0
0
Renal insufficiency a
17 (11)
17 (11)
7 (5)
7 (5)
Transfusion reactions a
17 (11)
16 (11)
3 (2)
1 (1)
Visual impairment (except bleeding) a
16 (11)
8 (5)
0
0
a Grouped terms: Hemorrhage: Anal hemorrhage, Blood blister, Blood urine present, Breast hematoma, Catheter site bruise, Catheter site hemorrhage, Central nervous system hemorrhage, Cerebral hematoma, Cerebral hemorrhage, Coagulopathy, Conjunctival hemorrhage, Contusion, Ecchymosis, Enterocolitis hemorrhagic, Epistaxis, Gastric hemorrhage, Gastrointestinal hemorrhage, Gingival bleeding, Hematemesis, Hematochezia, Hematoma, Hematuria, Hemoptysis, Hemorrhage, Hemorrhage intracranial, Hemorrhage subcutaneous, Hemorrhage urinary tract, Hemorrhoidal hemorrhage, Lip hematoma, Lip hemorrhage, Lower gastrointestinal hemorrhage, Melena, Mouth hemorrhage, Mucosal hemorrhage, Periorbital hematoma, Periorbital hemorrhage, Pharyngeal hematoma, Pharyngeal hemorrhage, Post procedural contusion, Post procedural hematoma, Post procedural hemorrhage, Pulmonary alveolar hemorrhage, Pulmonary hemorrhage, Purpura, Rectal hemorrhage, Retinal hemorrhage, Scleral hemorrhage, Scrotal hematoma, Skin ulcer hemorrhage, Small intestinal hemorrhage, Stomatitis hemorrhagic, Subdural hematoma, Subdural hemorrhage, Subgaleal hematoma, Tongue hemorrhage, Traumatic hematoma, Upper gastrointestinal hemorrhage, Urethral hemorrhage, Vaginal hemorrhage, Vessel puncture site hemorrhage, Vitreous hemorrhage; Rash: Dermatitis, Dermatitis acneiform, Dermatitis allergic, Dermatitis contact, Eczema, Erythema nodosum, Exfoliative rash, Psoriasis, Rash, Rash erythematous, Rash follicular, Rash generalized, Rash macular, Rash maculo-papular, Rash papular, Rash pruritic, Rash pustular, Skin exfoliation; Edema: Face edema, Fluid overload, Fluid retention, Generalized edema, Localized edema, Edema, Edema peripheral, Penile edema, Scrotal edema, Swelling, Swelling face; Diarrhea/Colitis: Cecitis, Colitis, Diarrhea, Enterocolitis, Ileitis, Neutropenic colitis, Enteritis, Enterocolitis; Mucositis: Anal erosion, Anorectal discomfort, Duodenitis, Gastric ulcer, Gastrointestinal inflammation, Gingival pain, Gingival swelling, Gingivitis, Glossodynia, Laryngeal inflammation, Lip ulceration, Mouth ulceration, Mucosal inflammation, Mucosal ulceration, Odynophagia, Edema mouth, Esophageal ulcer, Esophagitis, Oral mucosa erosion, Oral mucosal blistering, Oral mucosal erythema, Pharyngeal ulceration, Proctalgia, Proctitis, Rectal ulcer, Stomatitis, Tongue ulceration, Oropharyngeal pain, Oral pain, Oropharyngeal discomfort, Pharyngeal erythema; Musculoskeletal pain: Arthralgia, Back pain, Bone pain, Coccydynia, Limb discomfort, Musculoskeletal chest pain, Musculoskeletal pain, Myalgia, Neck pain, Pain in extremity, Pain in jaw; Abdominal pain: Abdominal pain, Abdominal distension, Abdominal pain upper, Abdominal discomfort, Abdominal pain lower, Abdominal tenderness; Cough: Cough, Productive Cough; Headache: Headache, Sinus Headache; Dyspnea: Acute respiratory distress syndrome, Acute respiratory failure, Bronchospasm, Dyspnea, Dyspnea exertional, Respiratory distress, Respiratory failure, Wheezing; Fatigue: Fatigue, Asthenia; Arrhythmia: Arrhythmia, Arrhythmia supraventricular, Atrial fibrillation, Atrial flutter, Atrial tachycardia, Atrioventricular block first degree, Atrioventricular block second degree, Bradycardia, Bundle branch block right, Extrasystoles, Heart rate increased, Nodal arrhythmia, Nodal rhythm, Sinus arrest, Sinus arrhythmia, Sinus bradycardia, Sinus tachycardia, Supraventricular tachycardia, Tachycardia, Ventricular extrasystoles, Ventricular tachycardia; Pneumonia (excluding fungal): Lung consolidation, Lung infection, Lung infiltration, Pneumonia, Pneumonia aspiration, Pneumonia bacterial, Pneumonia klebsiella, Pneumonia pseudomonas aeruginosa, Pneumonia viral; Sleep disorders: Abnormal dreams, Insomnia, Nightmare, Sleep apnea syndrome, Sleep disorder; Bacteremia (excluding sepsis): Bacillus test positive, Bacteremia, Bacteroides bacteremia, Corynebacterium test positive, Enterobacter bacteremia, Enterococcal bacteremia, Enterococcus test positive, Escherichia bacteremia, Klebsiella bacteremia, Pseudomonal bacteremia, Pseudomonas test positive, Staphylococcal bacteremia, Staphylococcus test positive, Stomatococcus test positive, Streptococcal bacteremia, Streptococcus test positive, Escherichia test positive, Klebsiella test positive; Vomiting: Retching, Vomiting; Hypotension: Hypotension, Orthostatic hypotension; Non-conduction cardiotoxicity: Acute coronary syndrome, Acute endocarditis, Acute myocardial infarction, Angina pectoris, Aortic valve incompetence, Cardiac arrest, Cardiac failure, Cardiac failure congestive, Cardiac murmur, Cardiogenic shock, Cardiomegaly, Cardiomyopathy, Cardiotoxicity, Cytotoxic cardiomyopathy, Diastolic dysfunction, Dilatation atrial, Dilatation ventricular, Ejection fraction decreased, Endocarditis, Left ventricular dysfunction, Mitral valve incompetence, Myocardial infarction, Pericardial effusion, Pericarditis, Restrictive cardiomyopathy, Right ventricular hypertrophy; Dizziness: Dizziness, Dizziness postural, Dizziness exertional; Fungal infection: Aspergillosis, Bronchopulmonary aspergillosis, Candida test positive, Candidiasis, Fungemia, Fungal infection, Fungal skin infection, Intertrigo candida, Lower respiratory tract infection fungal, Oral candidiasis, Pneumonia fungal, Pulmonary mycosis, Sinusitis fungal, Skin candida, Tinea cruris, Tinea infection, Vulvovaginal mycotic infection, Wound infection fungal, Zygomycosis, Mycotic aneurysm; Hypertension: Blood pressure increased, Hypertension; Hypoxia: Hypoxia, Oxygen saturation decreased; Upper respiratory infection (excluding fungal): Acute sinusitis, Chronic sinusitis, Increased upper airway secretion, Nasal congestion, Pharyngitis, Rhinitis, Rhinorrhea, Sinus congestion, Sinusitis, Sinusitis bacterial, Upper respiratory tract congestion, Upper respiratory tract infection, Upper-airway cough syndrome, Viral upper respiratory tract infection; Chest pain: Chest discomfort, Chest pain, Non-cardiac chest pain, Pleuritic pain; Catheter/device/injection site reaction: Catheter site discharge, Catheter site erosion, Catheter site erythema, Catheter site inflammation, Catheter site edema, Catheter site pain, Catheter site pruritus, Catheter site rash, Infusion site edema, Infusion site pain, Infusion site vesicles, Catheter site related reaction; Delirium: Cognitive disorder, Confusional state, Delirium; Pruritis: Anal pruritis, Ear pruritis, Pruritis, Pruritis generalized; Sepsis (excluding fungal): Enterobacter sepsis, Escherichia sepsis, Klebsiella sepsis, Neutropenic sepsis, Sepsis, Septic shock, Staphylococcal sepsis, Streptococcal sepsis, Urosepsis, Viral sepsis; Renal insufficiency: Acute prerenal failure, Azotemia, Oliguria, Renal failure, Renal failure acute, Renal failure chronic; Transfusion reactions: Allergic transfusion reaction, Febrile non-hemolytic transfusion reaction, Transfusion reaction; Visual impairment (except bleeding): Photophobia, Photopsia, Photosensitivity reaction, Retinal tear, Scintillating scotoma, Uveitis, Vision blurred, Visual acuity reduced, Visual impairment, Vitreous detachment, Vitreous floaters
b Adverse reactions were graded using NCI CTCAE version 3.0.
During the consolidation phase (both consolidation cycles pooled) the two most common adverse reactions on the VYXEOS arm are the same as those during induction, hemorrhagic events and febrile neutropenia. These occurred at lower rates in the pooled consolidation phase (43% and 29%, respectively), compared to the induction phase. All of the common adverse reactions (≥ 10% incidence in the VYXEOS arm) seen in the pooled consolidation phase were also seen in the induction phase. These occurred at lower incidence in the consolidation phase, with the exception of chills, dizziness and pyrexia, where the incidences were relatively similar across the induction and consolidation cycles.
Other notable adverse drug reactions that occurred in less than 10% of patients treated with VYXEOS during induction or consolidation included:
- •
- Ear and labyrinth disorders: Deafness, Deafness unilateral
- •
- Eye Disorders: Eye conjunctivitis, Dry eye, Eye edema, Eye swelling, Eye irritation, Eye pain, Ocular discomfort, Ocular hyperemia, Periorbital edema, Scleral hyperemia
- •
- Gastrointestinal disorders: Dyspepsia
- •
- Psychiatric disorders: Hallucinations
- •
- Respiratory, thoracic and mediastinal disorders: Pneumonitis
Laboratory Abnormalities
All patients developed severe neutropenia, thrombocytopenia, and anemia. See Table 4 for the incidences of Grade 3 thrombocytopenia and Grade 4 neutropenia that were prolonged in the absence of active leukemia.
Table 4: Prolonged Cytopenias for Patients in Study 1 Induction 1
Consolidation 1 b
VYXEOS
N=58
n (%)
7+3
N=34
n (%)
VYXEOS
N=48
n (%)
5+2
N=32
n (%)
Prolonged thrombocytopenia a
16 (28)
4 (12)
12 (25)
5 (16)
Prolonged neutropenia a
10 (17)
1 (3)
5 (10)
1 (3)
a Platelets < 50 Gi/L or neutrophils < 0.5 Gi/L lasting past cycle day 42 in the absence of active leukemia.
b Patients receiving at least 1 consolidation.
Grade 3-4 chemistry abnormalities occurring in greater than 5% of VYXEOS treated patients in Study 1 are presented in Table 5.
Table 5: Grade 3-4 a Chemistry Abnormalities ≥ 5% of VYXEOS Treated Patients in Study 1 Induction
Consolidation
VYXEOS
N=153
n (%)
7+3
N=151
n (%)
VYXEOS
N=49
n (%)
5+2
N=32
n (%)
Chemistry Abnormalities
Hyponatremia
21 (14)
20 (13)
3 (6)
0
Hypokalemia
14 (9)
19 (13)
3 (6)
2 (6)
Hypoalbuminemia
11 (7)
19 (13)
1 (2)
4 (13)
Hyperbilirubinemia
9 (6)
6 (4)
1 (2)
1 (3)
Alanine aminotransferase
7 (5)
8 (5)
0
1 (3)
a Graded using NCI CTCAE version 3.0.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of VYXEOS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
‑ Infusion-related reactions
-
7 DRUG INTERACTIONS
7.1 Cardiotoxic Agents
Concomitant use of cardiotoxic agents may increase the risk of cardiotoxicity. Assess cardiac function more frequently when VYXEOS is coadministered with cardiotoxic agents [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on anecdotal data of cytarabine in pregnant women and results of studies of daunorubicin and cytarabine in animals, VYXEOS can cause embryo-fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of VYXEOS, daunorubicin, or cytarabine in pregnant women. Daunorubicin and cytarabine are reproductive and developmental toxicants in multiple species (mice, rats, and/or dogs), starting at a dose that was approximately 0.02 times the exposure in patients at the recommended human dose on a mg/m2 basis [see Animal Data]. Patients should be advised to avoid becoming pregnant while taking VYXEOS. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential harm to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Cytarabine can cause fetal harm if a pregnant woman is exposed to the drug. Four anecdotal cases of major limb malformations have been reported in infants after their mothers received intravenous cytarabine, alone or in combination with other agents, during the first trimester.
Animal Data
A liposomal formulation of daunorubicin was administered to rats on gestation days 6 through 15 at 0.3, 1.0, or 2.0 mg/kg/day (about 0.04, 0.14, or 0.27 the recommended human dose on a mg/m2 basis) and produced severe maternal toxicity and embryolethality at 2.0 mg/kg/day and was embryotoxic and caused fetal malformations (anophthalmia, microphthalmia, incomplete ossification) at 0.3 mg/kg/day. Embryotoxicity was characterized by increased embryo-fetal deaths, reduced numbers of litters, and reduced litter sizes.
Cytarabine was teratogenic in mice (cleft palate, phocomelia, deformed appendages, skeletal abnormalities) when doses ≥ 2 mg/kg/day were administered IP during the period of organogenesis (about 0.06 times the recommended human dose on a mg/m2 basis), and in rats (deformed appendages) when 20 mg/kg was administered as a single IP dose on day 12 of gestation (about 1.2 times the recommended human dose on a mg/m2 basis). Single IP doses of 50 mg/kg in rats (about 3 times the recommended human dose on a mg/m2 basis) on day 14 of gestation reduced prenatal and postnatal brain size and permanent impairment of learning ability.
Cytarabine was embryotoxic in mice when administered during the period of organogenesis. Embryotoxicity was characterized by decreased fetal weight at 0.5 mg/kg/day (about 0.02 times the recommended human dose on a mg/m2 basis), and increased early and late resorptions and decreased live litter sizes at 8 mg/kg/day (about 0.24 times the recommended human dose on a mg/m2 basis).
8.2 Lactation
Risk Summary
There are no data on the presence of daunorubicin, cytarabine, or their metabolites in human milk, their effects on the breastfed infant, or their effects on milk production. Because of the potential for serious adverse reactions in breastfed infants, advise lactating women not to breastfeed during treatment with VYXEOS and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
VYXEOS can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating VYXEOS.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with VYXEOS and for 6 months after the last dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with VYXEOS and for 6 months after the last dose [see Nonclinical Toxicology (13.1)].
Inferrtility
Based on findings of daunorubicin and cytarabine in animals, male fertility may be compromised by treatment with VYXEOS [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of VYXEOS have been established in pediatric patients 1 year and older with newly diagnosed t AML or AML-MRC. The use of VYXEOS for this indication is supported by evidence of effectiveness from an adequate and well-controlled study in adults with data on safety from two single-arm trials, which included patients in the following age groups: 7 patients 1 year to less than 2 years old, 33 patients 2 years to less than 12 years old, 13 patients 12 years old to less than 17 years old. [see Clinical Pharmacology (12.3)]. No new safety signals were observed in pediatric patients in these two single-arm trials. No differences in safety were observed by age. The safety and effectiveness of VYXEOS in pediatric patients less than 1 year of age with newly-diagnosed t-AML or AML-MRC have not been established.
8.5 Geriatric Use
Of the 375 patients who received VYXEOS (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome in clinical studies, 57% were 65 years and over. No overall differences in safety were observed between these patients and younger patients, with the exception of bleeding events, which occurred more frequently in patients 65 years and older compared to younger patients (77% vs. 59%).
8.6 Renal Impairment
Dosage adjustment is not required for patients with mild (creatinine clearance [CLCR] 60 mL/min to 89 mL/min by Cockcroft Gault equation [C-G]), moderate (CLCR 30 mL/min to 59 mL/min) or severe (CLCR 15 mL/min to 29 mL/min) renal impairment. VYXEOS has not been studied in patients with end-stage renal disease on hemodialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Dosage adjustment is not required for patients with a bilirubin level less than or equal to 3 mg/dL. VYXEOS has not been studied in patients with bilirubin level greater than 3 mg/dL [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
VYXEOS (daunorubicin and cytarabine) liposome for injection is a combination of daunorubicin and cytarabine in a 1:5 molar ratio encapsulated in liposomes for intravenous administration. The liposome membrane is composed of distearoylphosphatidylcholine (DSPC), distearoylphosphatidylglycerol (DSPG), and cholesterol in a 7:2:1 molar ratio.
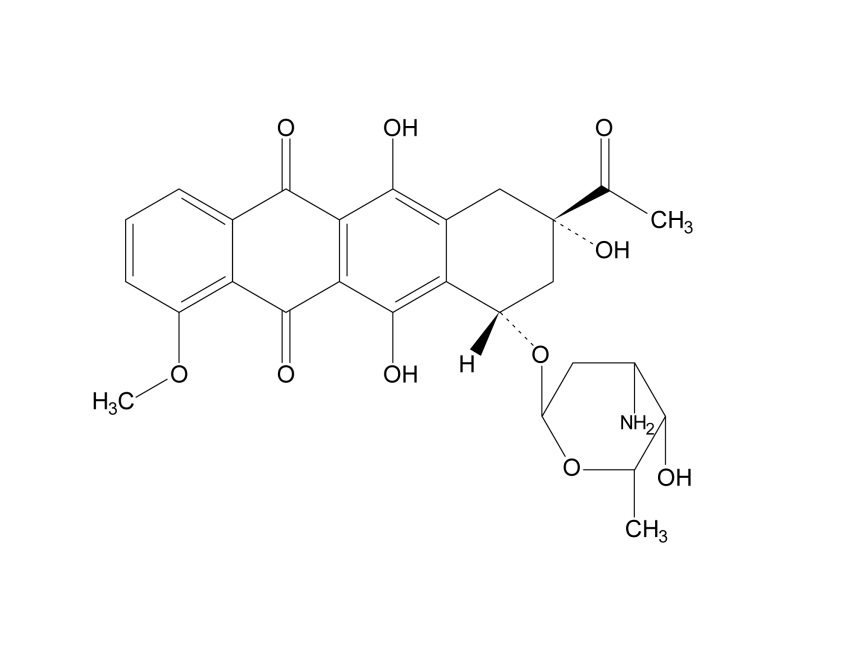
Daunorubicin is an anthracycline topoisomerase inhibitor. The chemical name for daunorubicin is (1S,3S)-3-acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside; its molecular weight is 527.52. Daunorubicin has the following structural formula:
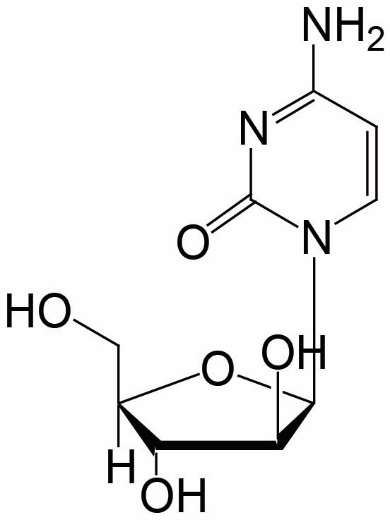
Cytarabine is a nucleoside metabolic inhibitor. The chemical name of cytarabine is 4-amino-1-β-D-arabinofuranosyl-2(1H)-pyrimidinone; its molecular weight is 243.22. Cytarabine has the following structural formula:
VYXEOS liposome for injection is supplied as a sterile, preservative-free, purple, lyophilized cake, in a single-dose vial. Each vial contains 44 mg daunorubicin and 100 mg cytarabine, and the following inactive ingredients: distearoylphosphatidylcholine 454 mg, distearoylphosphatidylglycerol 132 mg, cholesterol HP 32 mg, copper gluconate 100 mg, triethanolamine 4 mg, and sucrose 2054 mg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VYXEOS (daunorubicin and cytarabine) liposome for injection is a liposomal formulation of daunorubicin and cytarabine at a fixed 1:5 molar ratio. The 1:5 molar ratio of daunorubicin:cytarabine has been shown to have synergistic effects at killing leukemia cells in vitro and in murine models. Daunorubicin has antimitotic and cytotoxic activity, which is achieved by forming complexes with DNA, inhibiting topoisomerase II activity, inhibiting DNA polymerase activity, affecting regulation of gene expression, and producing DNA-damaging free radicals. Cytarabine is a cell cycle phase-specific antineoplastic agent, affecting cells only during the S-phase of cell division. Cytarabine acts primarily through inhibition of DNA polymerase. Based on animal data, the liposomes enter and persist in the bone marrow, where they are taken up intact by bone marrow cells. In leukemia-bearing mice, the liposomes are taken up by leukemia cells to a greater extent than by normal bone marrow cells. After cellular internalization, liposomes undergo degradation releasing cytarabine and daunorubicin within the intracellular environment.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At the therapeutic exposures with the recommended dosing regimen, no large mean changes in the QTc interval (i.e., > 20 msecs) were observed. An exposure-QTc analysis suggested no concentration-dependent QTc interval prolongation.
12.3 Pharmacokinetics
The pharmacokinetics of daunorubicin and cytarabine administered as VYXEOS were investigated in adult patients who received a dose of daunorubicin 44 mg/m2 and cytarabine 100 mg/m2 administered as a 90-minute intravenous infusion on days 1, 3, and 5. The pharmacokinetics of each drug was based on total plasma concentrations (i.e., encapsulated plus unencapsulated drug).
Following the dose administered on day 5, the mean (% coefficient of variation [CV]) maximum plasma concentration (Cmax) for daunorubicin was 26.0 (32.7%) mcg/mL and cytarabine was 62.2 (33.7%) mcg/mL. The mean (%CV) area under the curve (AUC) during one dosing interval for daunorubicin was 637 (38.4%) mcg∙h/mL and cytarabine was 1900 (44.3%) mcg∙h/mL.
The accumulation ratio was 1.3 for daunorubicin and 1.4 for cytarabine. There was no evidence of time-dependent kinetics or major departures from dose proportionality over the range of 1.3 mg/3 mg per m2 to 59 mg/134 mg per m2 (0.03 to 1.3 times the approved recommended dosage).
Distribution
The volume of distribution (%CV) for daunorubicin is 6.6 L (36.8%) and cytarabine is 7.1 L (49.2%). Plasma protein binding was not evaluated.
Elimination
VYXEOS exhibits a prolonged half-life (%CV) of 31.5 h (28.5%) for daunorubicin and 40.4 h (24.2%) for cytarabine with greater than 99% of the daunorubicin and cytarabine in the plasma remaining encapsulated within the liposomes. The clearance (%CV) is 0.16 L/h (53.3%) for daunorubicin and 0.13 L/h (60.2%) for cytarabine.
Metabolism
Subsequent to release from VYXEOS liposomes, daunorubicin is catalyzed by aldoketo reductase and carbonyl reductase enzymes to the active metabolite daunorubicinol. Cytarabine is metabolized by cytidine deaminase to the inactive metabolite 1-β-D-arabinofuranosyluracil (AraU).
Excretion
Urinary excretion of daunorubicin and daunorubicinol accounts for 9% of the administered dose of daunorubicin, and urinary excretion of cytarabine and AraU accounts for 71% of the administered dose of cytarabine.
Specific Populations
No clinically meaningful effects on the pharmacokinetics of daunorubicin and cytarabine were observed based on age (1 to 81 years), sex, race, body weight (9 to 156 kg), body mass index (14 to 48 kg/m2), and white blood cell count (0.2 to 111 Gi/L) after adjusting dose by body surface area.
Pediatric Patients
The exposures of total daunorubicin and cytarabine observed in pediatric patients were within the values observed in adults given the same dose based on body surface area.
Geriatric Patients
The exposures of total daunorubicin and cytarabine observed in patients aged >65 years were within the values observed in patients aged 18 to 64 years given the same dose based on body surface area.
Patients with Renal Impairment
Comparing to patients with normal renal function (n=7, CLcr ≥ 90 mL/min), patients with moderate renal impairment (n=8, CLcr 30 to 59 mL/min) showed 8% and 6% decrease, respectively, in the mean AUCtau of total cytarabine and daunorubicin; patients with severe renal impairment (n=6, CLcr 15 to 29 mL/min) showed 0.4% increase and 3% decrease, respectively, in the mean AUCtau of cytarabine and daunorubicin in a dedicated study.
There are no data in patients with end-stage renal disease managed with hemodialysis.
Patients with Hepatic Impairment
The pharmacokinetics of total cytarabine and daunorubicin were not altered in patients with bilirubin less than and equal to 3 mg/dL. The pharmacokinetics in patients with bilirubin greater than 3 mg/dL is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and impairment of fertility studies with VYXEOS (daunorubicin and cytarabine) liposome for injection have not been conducted.
Carcinogenicity and mutagenicity studies have been conducted with daunorubicin. Published literature reported data that suggest daunorubicin (5 mg/kg) could be tumorigenic in rats at 0.68 times the recommended human dose on an mg/m2 basis. A high incidence of mammary tumors was observed about 120 days after a single intravenous dose of 12.5 mg/kg daunorubicin in rats (about 1.7 times the recommended human dose on an mg/m2 basis). A carcinogenic evaluation of daunorubicin by the IARC Working Group classified daunorubicin as a possible human carcinogen based on sufficient evidence in animals and inadequate data in humans. Daunorubicin was mutagenic in in vitro tests (Ames assay, V79 hamster cell assay), and clastogenic in in vitro (CCRF-CEM human lymphoblasts) and in in vivo (SCE assay in mouse bone marrow) tests.
Daunorubicin intravenous doses of 0.25 mg/kg/day (about 0.12 times the recommended human dose on a mg/m2 basis) in male dogs caused testicular atrophy and total aplasia of spermatocytes in the seminiferous tubules.
Cytarabine was mutagenic in in vitro tests and was clastogenic in vitro (chromosome aberrations and SCE in human leukocytes) and in vivo (chromosome aberrations and SCE assay in rodent bone marrow, mouse micronucleus assay). Cytarabine caused the transformation of hamster embryo cells and rat H43 cells in vitro.
No studies assessing the impact of cytarabine on fertility are available in the literature. Cytarabine was clastogenic to meiotic cells; a dose-dependent increase in sperm-head abnormalities and chromosomal aberrations occurred in mice given IP cytarabine.
-
14 CLINICAL STUDIES
Study 1
Study 1 (NCT01696084) was a randomized, multicenter, open-label, active-controlled study which compared VYXEOS to a standard combination of cytarabine and daunorubicin (7+3) in patients 60 to 75 years of age with newly diagnosed t-AML or AML-MRC. The patients were randomized (1:1) and stratified by age and AML sub-type to receive VYXEOS or 7+3 for induction and consolidation. VYXEOS (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) liposome was given intravenously on days 1, 3, and 5 for the first induction and on days 1 and 3 for the second induction if needed. For consolidation, the VYXEOS dose was (daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) liposome on days 1 and 3. In the 7+3 arm, first induction consisted of cytarabine 100 mg/m2/day on days 1 through 7 by continuous infusion and daunorubicin 60 mg/m2/day on days 1, 2, and 3; for second induction and consolidation cycles, cytarabine 100 mg/m2/day was given on days 1 through 5 and daunorubicin 60 mg/m2/day on days 1 and 2. Patients could receive up to 2 cycles of induction and 2 cycles of consolidation in each arm. A second induction was highly recommended for patients who did not achieve a response and was mandatory for patients achieving greater than 50% reduction in percent blasts. Post remission therapy with hematopoietic stem cell transplantation (HSCT) was permitted either in place of or after consolidation chemotherapy.
There were 153 patients randomized to VYXEOS and 156 patients randomized to the 7+3 control arm. The randomized patients had a median age of 68 (range, 60-75 years), 61% were male, and 88% had an ECOG performance status of 0–1. Twenty percent had t-AML, 54% had AML with an antecedent hematological disorder, and 25% had de novo AML with myelodysplasia-related cytogenetic abnormalities. Thirty-four percent had been treated previously with a hypomethylating agent for MDS. Fifty-four percent of patients with cytogenetics data had an adverse karyotype. The demographic and baseline disease characteristics were generally balanced between the study arms. FLT3 mutation was identified in 15% (43/279) of patients tested, and NPM1 mutation was identified in 9% (25/283) patients tested.
All patients on the VYXEOS arm and 97% of those on the control arm received at least 1 cycle of induction, and 32% on the VYXEOS arm and 21% on the control arm received at least 1 cycle of consolidation. The rate of HSCT in first CR was 20% in the VYXEOS arm and 12% in the control arm; the overall rate of HSCT (induction failure, first CR, or as salvage after relapse) was 34% (52/153) in the VYXEOS arm and 25% (39/156) on the control arm.
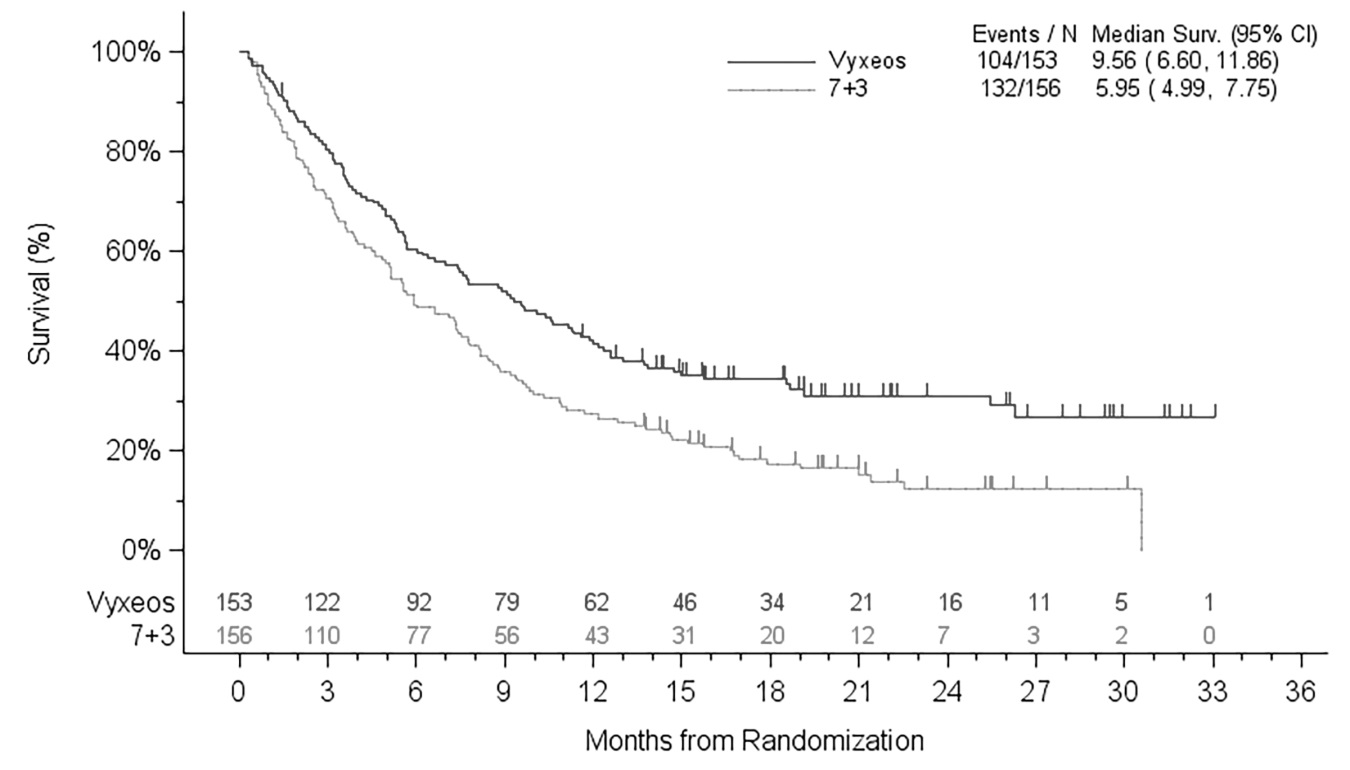
Efficacy was established on the basis of overall survival from the date of randomization to death from any cause. VYXEOS demonstrated superiority in overall survival compared with the 7+3 control (Figure 1). The efficacy results are shown in Table 6.
Figure 1: Kaplan-Meier Curve for Overall Survival, ITT Population
Table 6: Efficacy Results for Study 1 VYXEOS
N=153
7+3
N=156
Overall Survival
Median survival, months (95% CI)
9.6 (6.6, 11.9)
5.9 (5.0, 7.8)
Hazard ratio (95% CI)
0.69 (0.52, 0.90)
p–value (2–sided) a
0.005
Complete Response Rate
CR, n (%)
58 (38)
41 (26)
p-value (2–sided) b
0.036
Abbreviations: CI = Confidence interval; CR = Complete Remission
a p-value from stratified log rank test stratifying by age and AML sub-type
b p-value from Mantel-Haenszel test stratifying by age and AML sub-type
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VYXEOS (daunorubicin and cytarabine) liposome for injection is supplied as a sterile, preservative-free, purple, lyophilized cake, in a single-dose vial. Each VYXEOS vial (NDC 68727-745-01) contains 44 mg daunorubicin and 100 mg cytarabine.
NDC 68727-745-02: Carton containing 2 vials of VYXEOS
Storage
Store unreconstituted VYXEOS vials in a refrigerator at 2°C to 8°C (36°F to 46°F) in an upright position. The vial should be stored in its original carton to protect from light.
Handling and Disposal
VYXEOS is a hazardous drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Hemorrhage
Inform patients of the risk of fatal bleeding. Advise patients of the need for periodic monitoring of blood counts and of the importance of keeping scheduled appointments for blood work and necessary transfusions. Advise patients to contact a healthcare provider for new onset fever or symptoms of infection or if they notice signs of bruising or bleeding [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Cardiotoxicity
Advise patients to contact their healthcare provider if they develop symptoms of heart failure [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform patients of the risk of hypersensitivity reactions, including anaphylaxis. Describe the symptoms of hypersensitivity reactions, including anaphylaxis, and instruct the patient to seek medical attention immediately if they experience such symptoms [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
VYXEOS can cause fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of VYXEOS and to inform their healthcare provider of a known or suspected pregnancy before and during treatment with VYXEOS [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)and (8.3)].
Lactation
Advise patients not to breastfeed during treatment with VYXEOS and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males of reproductive potential that VYXEOS may cause temporary or permanent infertility [see Use in Specific Populations (8.3)].
Concomitant Medications
Advise patients to speak with their physicians about any other medication they are currently taking [see Drug Interactions (7)].
This product's label may have been updated. For full prescribing information, please visit labels.fda.gov.
Distributed by:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94304
Protected by U.S. Patent Nos. 7,238,367; 7,744,921; 7,850,990; 8,022,279; 8,092,828; 8,431,806; 8,518,437; 9,271,931; 10,028,912; 10,166,184; and 10,835,492
VYXEOS® is a trademark of Jazz Pharmaceuticals plc or its subsidiaries.
©2022 Jazz Pharmaceuticals www.vyxeos.com
- PACKAGE/LABEL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VYXEOS
(daunorubicin and cytarabine) liposome injection, powder, lyophilized, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68727-745 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYTARABINE (UNII: 04079A1RDZ) (CYTARABINE - UNII:04079A1RDZ) CYTARABINE 100 mg in 20 mL DAUNORUBICIN (UNII: ZS7284E0ZP) (DAUNORUBICIN - UNII:ZS7284E0ZP) DAUNORUBICIN 44 mg in 20 mL Inactive Ingredients Ingredient Name Strength DISTEAROYLPHOSPHATIDYLCHOLINE, DL- (UNII: EAG959U971) 454 mg in 20 mL DISTEAROYLPHOSPHATIDYLGLYCEROL, DL- (UNII: 4271ZA8WXO) 132 mg in 20 mL CHOLESTEROL (UNII: 97C5T2UQ7J) 32 mg in 20 mL COPPER GLUCONATE (UNII: RV823G6G67) 100 mg in 20 mL TROLAMINE (UNII: 9O3K93S3TK) 4 mg in 20 mL SUCROSE (UNII: C151H8M554) 2054 mg in 20 mL Product Characteristics Color PURPLE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68727-745-05 100 mL in 1 CARTON; Type 0: Not a Combination Product 08/03/2017 09/27/2022 2 NDC:68727-745-02 40 mL in 1 CARTON; Type 0: Not a Combination Product 08/03/2017 3 NDC:68727-745-01 20 mL in 1 VIAL; Type 0: Not a Combination Product 08/03/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209401 08/03/2017 Labeler - Jazz Pharmaceuticals, Inc. (135926363) Establishment Name Address ID/FEI Business Operations Baxter Oncology GmbH 344276063 MANUFACTURE(68727-745) , ANALYSIS(68727-745) , PACK(68727-745) Establishment Name Address ID/FEI Business Operations Jazz Pharmaceuticals Ireland Limited 986019606 MANUFACTURE(68727-745) , ANALYSIS(68727-745) Establishment Name Address ID/FEI Business Operations Jazz Pharmaceuticals Ireland 896650210 MANUFACTURE(68727-745) Establishment Name Address ID/FEI Business Operations Almac Sciences Ireland Limited 985822621 ANALYSIS(68727-745) Establishment Name Address ID/FEI Business Operations Charles Rivers Laboratories Ireland Limited 988712659 ANALYSIS(68727-745) Establishment Name Address ID/FEI Business Operations Eurofins BioPharma Product Testing Ireland Limited 238239933 ANALYSIS(68727-745) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories Inc. 069777290 ANALYSIS(68727-745) Establishment Name Address ID/FEI Business Operations Millmount Healthcare Limited 986018132 LABEL(68727-745) , PACK(68727-745)