Label: CHOLETEC- mebrofenin injection, powder, lyophilized, for solution
- NDC Code(s): 0270-0083-20
- Packager: Bracco Diagnostics Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture of 45 mg mebrofenin, 0.54 mg (minimum) stannous fluoride dihydrate, SnF2•2H2O and 1.03 mg total tin, maximum (as stannous fluoride dihydrate, SnF2•2H2O), not more than 5.2 mg methylparaben, and 0.58 mg propylparaben. The pH is adjusted with sodium hydroxide or hydrochloric acid prior to lyophilization. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture.
The pH of the reconstituted product is 4.2 to 5.7.
The structure of mebrofenin (2,2’-[[2-[(3-Bromo-2,4,6-trimethylphenyl)-amino]-2-oxoethyl]imino] bisacetic acid) is shown below:
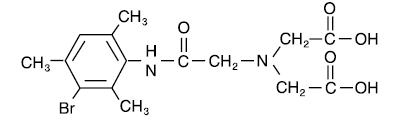
When sterile, pyrogen-free sodium pertechnetate Tc 99m injection is added to the vial, the diagnostic agent Technetium Tc 99m Mebrofenin is formed for administration by intravenous injection.
-
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1 The principal photon that is useful for detection and imaging studies is listed in Table 1.
TABLE 1 1Kocher, David C., “Radioactive Decay Data Tables”, DOE/ TIC-11026, (1981) p.108. Principal Radiation Emission Data Radiation Mean % per
DisintegrationMean Energy
(keV)Gamma-2 89.07 140.5 External Radiation
The specific gamma ray constant for Tc 99m is 0.78 R/hour-millicurie at 1 cm. The first half value layer is 0.017 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
TABLE 2 Radiation Attenuation by Lead Shielding Shield Thickness
(Pb) cmCoefficient
of Attenuation0.017 0.5 0.08 10-1 0.16 10-2 0.25 10-3 0.33 10-4 To correct for physical decay of technetium Tc 99m, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
TABLE 3 *Calibration time Physical Decay Chart: Tc 99m half-life 6.02 hours Hours Fraction
RemainingHours Fraction
Remaining0* 1.000 7 0.447 1 0.891 8 0.398 2 0.794 9 0.355 3 0.708 10 0.316 4 0.631 11 0.282 5 0.562 12 0.251 6 0.501 18 0.126 -
CLINICAL PHARMACOLOGY
Mebrofenin is an iminodiacetic acid (HIDA) derivative with no known pharmacologic action at the recommended doses.
Following intravenous administration in normal subjects, Technetium Tc 99m Mebrofenin was rapidly cleared from the circulation. The mean percent injected dose remaining in the blood at 10 minutes was 17%. The injected activity was cleared through the hepatobiliary system with visualization of the liver by 5 minutes and maximum liver uptake occurring at 11 minutes post-injection. Hepatic duct and gallbladder visualization occurred by 10 to 15 minutes and intestinal activity was visualized by 30 to 60 minutes in subjects with normal hepatobiliary function. The mean percent injected dose excreted in the urine during the first 3 hours was 1% (0.4 to 2.0%).
Elevated serum bilirubin levels increase renal excretion of Tc 99m HIDA agents. In two studies in which Tc 99m Mebrofenin was administered to patients having mean elevated serum bilirubin levels of 9.8 mg/dL (1.7 to 46.3 mg/dL), the mean percent injected dose excreted in the urine during the first 3 hours was 3% (0.2 to 11.5%). The mean percent injected dose excreted in the urine during 3-24 hours was 14.9% (0.4 to 34.8%).
In jaundiced patients, the percent injected dose remaining in the blood at 10 minutes may be twice as high or more than the level in normals. Hepatobiliary transit may be delayed and visualization times increased. As a consequence, the quality of the images obtained frequently diminishes.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Contents of the reaction vial are intended only for use in the preparation of Technetium Tc 99m Mebrofenin and are not to be administered directly to the patient.
Delayed or non-visualization of the gallbladder may occur in the immediate post-prandial period or after prolonged fasting or parenteral feeding. Functional biliary obstruction may accompany chronic cholecystitis or pancreatitis. In addition, patients with hepatocellular disease may show non-visualization or delayed visualization of the gallbladder. Delayed intestinal transit may also be noted in such patients. Juvenile hepatitis may be associated with gallbladder non-visualization and the failure to visualize activity in the intestine. Administration of meperidine or morphine may delay intestinal transit of the imaging agent and may result in nonvisualization. Septic patients may show absent or delayed hepatobiliary clearance. Thus, a positive finding does not of itself permit a differential diagnosis of any of the above conditions and should be evaluated in the light of the total clinical picture and results of other diagnostic modalities.
The components of the kit are supplied sterile and nonpyrogenic. Aseptic procedures normally employed in making additions and withdrawals from sterile, nonpyrogenic containers should be used during the addition of the pertechnetate solution and the withdrawal of doses for patient administration.
The Technetium Tc 99m labeling reactions involved in preparing the agent depend on maintaining the stannous ion in the reduced state. Any oxidant present in the sodium pertechnetate Tc 99m supply may, thus, adversely affect the quality of the radiopharmaceutical. Hence, sodium pertechnetate Tc 99m containing oxidants should not be employed.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides.
As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient consistent with proper patient management and to ensure minimum radiation exposure to occupational workers.
Tc 99m Mebrofenin should be formulated no more than 18 hours prior to clinical use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Mebrofenin may affect fertility in males or females.
Pregnancy
Animal reproduction studies have not been conducted with Technetium Tc 99m Mebrofenin. It is also not known whether Technetium Tc 99m Mebrofenin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Technetium Tc 99m Mebrofenin should be given to a pregnant woman only if the expected benefits to be gained clearly outweigh the potential hazards.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The suggested intravenous dose range of Technetium Tc 99m Mebrofenin in the average patient (70 kg) is:
Nonjaundiced patient: 74-185 MBq (2-5 mCi) Patient with serum
bilirubin level greater
than 1.5 mg/dL:111-370 MBq (3-10 mCi) The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
The patient should be in a fasting state, 4 hours is preferable. False positives (non-visualization) may result if the gallbladder has been emptied by ingestion of food.
An interval of at least 24 hours should be allowed before repeat examination.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
-
RADIATION DOSIMETRY
The estimated absorbed radiation doses 1,2 to organs and tissues of an average subject (70 kg) from an intravenous injection of 370 MBq (10 millicuries) of Technetium Tc 99m Mebrofenin are shown in Table 4.
TABLE 4 †Method of Calculation:
(1) Loberg, M.D., Buddemeyer, E.V.: Application of pharmacokinetic modeling to the radiation dosimetry of hepatobiliary agents. In Third International Radiopharmaceutical Dosimetry Symposium, FDA No. 81-8166, U.S. Department of Health and Human Services, Public Health Service, FDA, Bureau of Radiological Health, Rockville, MD, (1981) pp. 318-332.
(2) Values for S: “S”, Absorbed Dose per Unit Cumulated Activity for Selected Radionuclides and Organs, MIRD Pamphlet No. 11 (1975).
* Bilirubin <1.5 mg/dL
Calculations assume that 98% of the injected activity is taken up by the liver; activity not removed in the urine in 24 hours is excreted in the intestines and no enterohepatic circulation of activity.
**
Bilirubin >10 mg/dL (mean 21.8 mg/dL)
Calculations assume that 66% of the injected activity is taken up by the liver; activity not removed in the urine in 24 hours is excreted in the intestines and no enterohepatic circulation of activity.Estimated Absorbed Radiation Doses† Normal Subjects* Severely
Jaundiced Patients**Tissue mGy/
370 MBqrads/
10 mCimGy/
370 MBqrads/
10 mCiTotal Body 2.0 0.2 1.7 0.17 Liver 4.7 0.47 8.1 0.81 Gallbladder Wall 13.7 1.37 12.5 1.25 Small Intestine 29.9 2.99 16.0 1.60 Upper Large Intestine Wall 47.4 4.74 24.8 2.48 Lower Large Intestine Wall 36.4 3.64 19.7 1.97 Kidney 2.2 0.22 1.9 0.19 Urinary Bladder Wall 2.9 0.29 24.2 2.42 Ovaries 10.1 1.01 6.4 0.64 Testes 0.5 0.05 1.1 0.11 Red Marrow 3.4 0.34 2.5 0.25 -
HOW SUPPLIED
Choletec (Kit for the Preparation of Technetium Tc 99m Mebrofenin) is supplied in kits of 10 reaction vials. Each vial contains a sterile, nonpyrogenic lyophilized mixture of 45 mg mebrofenin, 0.54 mg (minimum) stannous fluoride dihydrate, SnF2•2H2O and 1.03 mg total tin, maximum (as stannous fluoride dihydrate, SnF2•2H2O), not more than 5.2 mg methylparaben, and 0.58 mg propylparaben. The pH has been adjusted with hydrochloric acid or sodium hydroxide prior to lyophilization. The lyophilized vial contents are sealed under nitrogen at the time of manufacture. The pH of the reconstituted product is 4.2 to 5.7.
Kit Contents
10 sterile multidose reaction vials.
20 pressure-sensitive labels for Technetium Tc 99m Mebrofenin.
1 package insert.Preparation
Preparation of Technetium Tc 99m Mebrofenin is done by the following aseptic procedure:
- Waterproof gloves should be worn during the preparation procedure.
- Place reaction vial in an appropriate lead shield.
- Swab the rubber closure of the reaction vial with a germicide.
- Inject 1 to 5 mL sterile additive free sodium pertechnetate Tc 99m injection containing up to 3700 MBq (100 mCi) Tc 99m into the reaction vial. Be sure to maintain a nitrogen atmosphere in the vial by not introducing air during reconstitution.
NOTE: If sodium pertechnetate Tc 99m injection must be diluted for use with Choletec (Kit for the preparation of Technetium Tc 99m Mebrofenin), only preservative free Sodium Chloride Injection USP should be used. - Secure the lead shield cover. Swirl the vial gently to mix contents and let stand for 15 minutes.
- Record the date and time of preparation on pressure-sensitive label.
- Affix pressure-sensitive label to shield.
- Examine vial contents. If the solution is not clear and free of particulate matter and discoloration on visual inspection, it should not be used.
- Measure the radioactivity by a suitable calibration system and record on the shield label prior to patient administration.
- Withdraw material with a sterile lead shielded syringe for use within 18 hours of preparation.
Storage
Store the kit as supplied at 20-25°C (68-77°F) [See USP] prior to and following reconstitution. Use within 18 hours of reconstitution.
The U.S. Nuclear Regulatory Commission has approved this reagent kit for distribution to persons licensed to use byproduct material identified in §35.200 of 10 CFR Part 35, to persons who hold an equivalent license issued by an Agreement State, and, outside the United States, to persons authorized by the appropriate authority.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHOLETEC
mebrofenin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0270-0083 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEBROFENIN (UNII: 7PV0B6ED98) (MEBROFENIN - UNII:7PV0B6ED98) MEBROFENIN 45 mg Inactive Ingredients Ingredient Name Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) 0.54 mg METHYLPARABEN (UNII: A2I8C7HI9T) 5.2 mg PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.58 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0270-0083-20 10 in 1 CARTON 01/21/1987 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018963 01/21/1987 Labeler - Bracco Diagnostics Inc (849234661) Registrant - Bracco Diagnostics Inc (849234661) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier LLC 069263643 MANUFACTURE(0270-0083) , ANALYSIS(0270-0083) Establishment Name Address ID/FEI Business Operations Sigma-Aldrich Production GmbH 480945463 API MANUFACTURE(0270-0083) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, Inc 069777290 ANALYSIS(0270-0083) Establishment Name Address ID/FEI Business Operations GE Healthcare Inc. (Medi-Physics Inc. dba GE Healthcare) 095263729 ANALYSIS(0270-0083)




