Label: BYFAVO- remimazolam besylate injection, powder, lyophilized, for solution
- NDC Code(s): 71390-011-00, 71390-011-11
- Packager: Acacia Pharma, Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BYFAVO safely and effectively. See full prescribing information for BYFAVO.
BYFAVO® (remimazolam) for injection, for intravenous use, CIV
Initial U.S. Approval: 2020WARNING: PERSONNEL AND EQUIPMENT FOR MONITORING AND RESUSCITATION, AND RISKS FROM CONCOMITANT USE WITH OPIOID ANALGESICS AND OTHER SEDATIVE-HYPNOTICS
See full prescribing information for complete boxed warning
- Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO. (2.1, 5.1)
- Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation. (2.1, 5.1)
- BYFAVO has been associated with hypoxia, bradycardia, and hypotension. Continuously monitor vital signs during sedation and through the recovery period. (2.1, 5.1)
- Resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO. (2.1, 5.1)
- Concomitant use of benzodiazepines with opioid analgesics may result in profound sedation, respiratory depression, coma, and death. The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including other benzodiazepines and propofol. Continuously monitor patients for respiratory depression and depth of sedation. (5.2, 7.1)
RECENT MAJOR CHANGES
Warnings and Precautions (5.4) 01/2023 INDICATIONS AND USAGE
BYFAVO (remimazolam) for injection is a benzodiazepine indicated for the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less. (1)
DOSAGE AND ADMINISTRATION
Individualize and titrate BYFAVO dosing to desired clinical effect. (2.2)
Adult Patients:
- Administer an initial dose intravenously as a 5 mg push injection over a 1-minute time period. (2.2)
- If necessary, administer supplemental doses of 2.5 mg intravenously over a 15-second time period. At least 2 minutes must elapse prior to the administration of any supplemental dose. (2.2)
ASA III-IV Patients (at the discretion of the physician):
- Based on the general condition of the patient, administer 2.5 mg to 5 mg over 1-minute time period. (2.2)
- If necessary, administer supplemental doses of 1.25 mg to 2.5 mg intravenously over a 15-second time period. At least 2 minutes must elapse prior to the administration of any supplemental dose. (2.2)
DOSAGE FORMS AND STRENGTHS
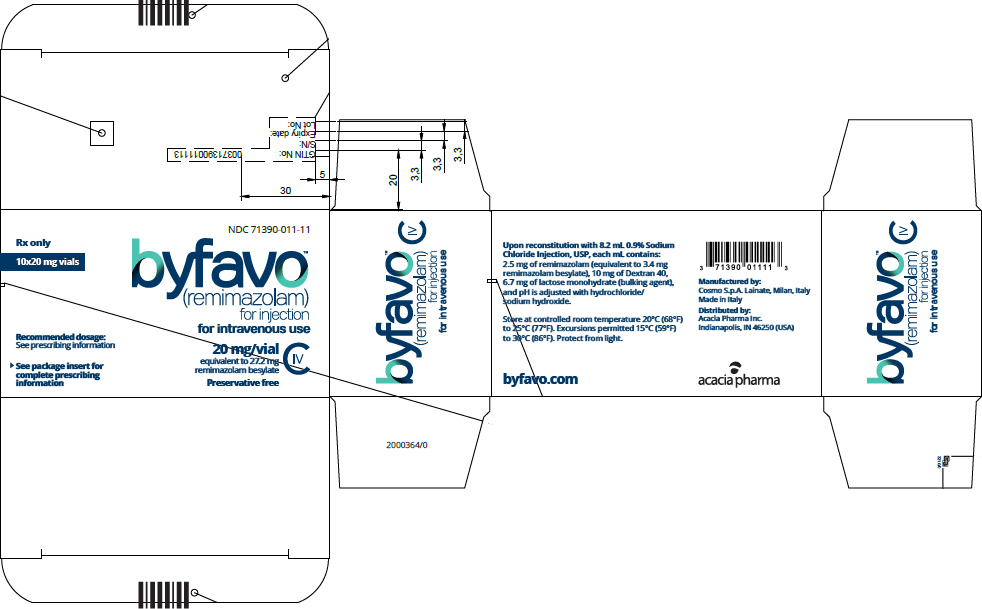
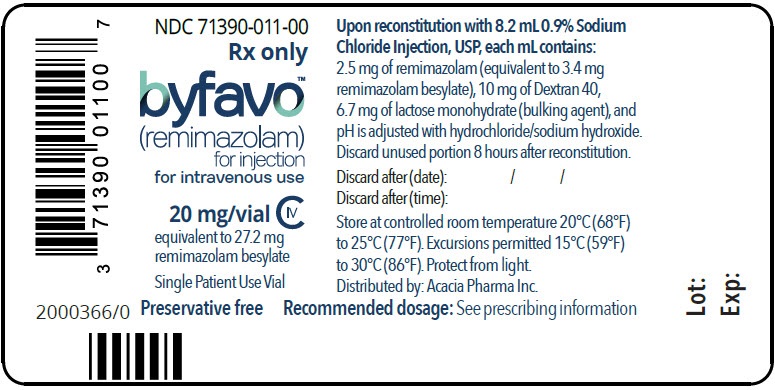
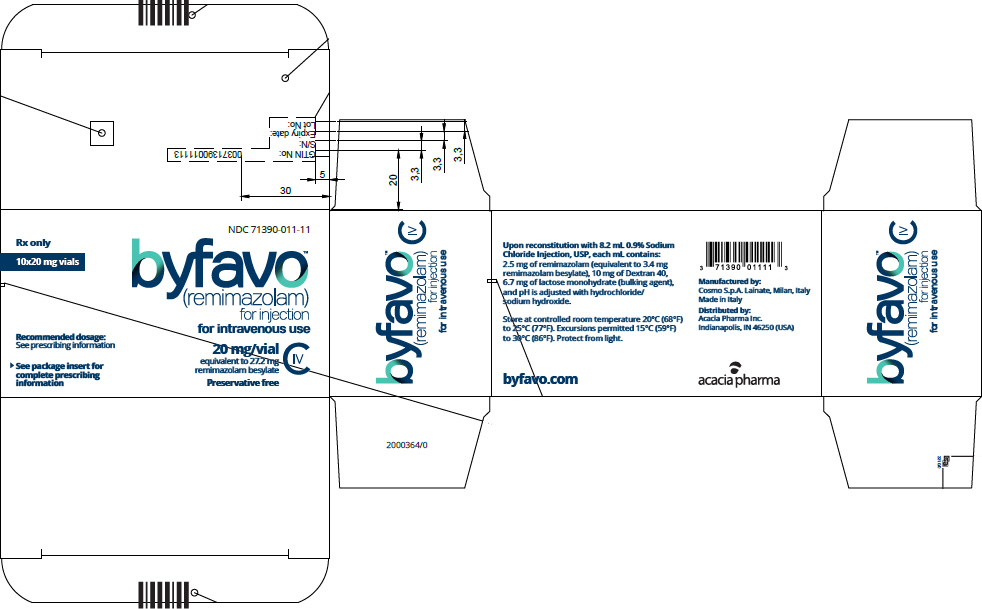
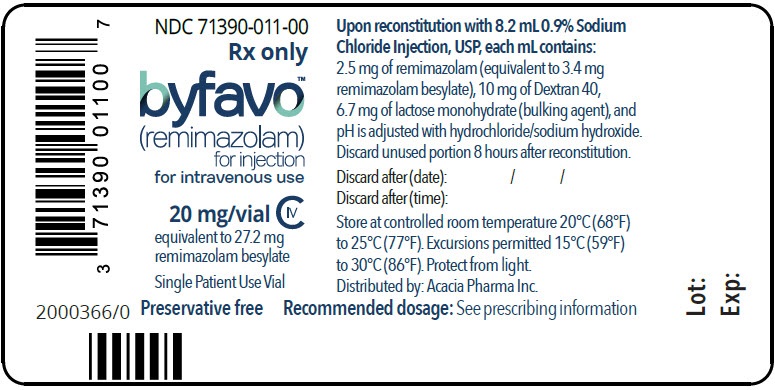
Each glass, single-patient-use vial contains 20 mg BYFAVO (remimazolam) lyophilized powder for reconstitution, equivalent to 27.2 mg remimazolam besylate. (3)
CONTRAINDICATIONS
Hypersensitivity to dextran 40. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: Hypersensitivity reactions including anaphylaxis may occur. (5.3)
Neonatal Sedation and Withdrawal Syndrome: Receiving benzodiazepines during pregnancy can result in neonatal sedation and/or neonatal withdrawal. (5.4, 8.1)
Pediatric Neurotoxicity: In developing animals, exposures greater than 3 hours cause neurotoxicity. Weigh benefits against potential risks when considering elective procedures in children under 3 years old. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (>10%) in patients receiving BYFAVO for procedural sedation are hypotension, hypertension, diastolic hypertension, systolic hypertension, hypoxia, and diastolic hypotension. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Acacia Pharma at 1-877-357-9237 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Lactation: A lactating woman may pump and discard breast milk for 5 hours after treatment with BYFAVO. (8.2)
Pediatric Use: BYFAVO should not be used in patients less than 18 years of age. (8.4)
Geriatric Use: Sedating drugs, such as BYFAVO, may cause confusion and over-sedation in the elderly; elderly patients generally should be observed closely. (8.5)
Severe Hepatic Impairment: In patients with severe hepatic impairment the dose of BYFAVO should be carefully titrated to effect. Depending on the overall status of the patient, reduced doses might be indicated. (8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: PERSONNEL AND EQUIPMENT FOR MONITORING AND RESUSCITATION AND RISKS FROM CONCOMITANT USE WITH OPIOID ANALGESICS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Basic Dosing Information
2.3 Preparation
2.4 Administration with Other Fluids
3 DOSAGE FORM AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Personnel and Equipment for Monitoring and Resuscitation
5.2 Risks from Concomitant Use with Opioid Analgesics and Other Sedative-Hypnotics
5.3 Hypersensitivity Reactions
5.4 Neonatal Sedation and Withdrawal Syndrome
5.5 Pediatric Neurotoxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Opioid Analgesics and Other Sedative-Hypnotics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Colonoscopy Study 1 (NCT 02290873)
14.2 Bronchoscopy Study (NCT 02296892)
14.3 Colonoscopy Study 2 (NCT 02532647)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: PERSONNEL AND EQUIPMENT FOR MONITORING AND RESUSCITATION AND RISKS FROM CONCOMITANT USE WITH OPIOID ANALGESICS
Personnel and Equipment for Monitoring and Resuscitation
- Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
- Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
- BYFAVO has been associated with hypoxia, bradycardia, and hypotension. Continuously monitor vital signs during sedation and during the recovery period [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
- Resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
Risks From Concomitant Use With Opioid Analgesics and Other Sedative-Hypnotics
Concomitant use of benzodiazepines, including BYFAVO, and opioid analgesics may result in profound sedation, respiratory depression, coma, and death. The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including other benzodiazepines and propofol. Continuously monitor patients for respiratory depression and depth of sedation [see Warnings and Precautions (5.2), Drug Interactions (7.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
BYFAVO can depress respiration. Continuously monitor patients for early signs of hypoventilation, airway obstruction, and apnea using capnography, pulse oximetry, and clinical assessment.
Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO.
Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation.
Supplemental oxygen, resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO. A benzodiazepine reversal agent should be immediately available.
Continuously monitor vital signs during sedation and through the recovery period [see Warnings and Precautions (5.1)].
Peak sedation occurs approximately 3 to 3.5 minutes after an initial 5 mg intravenous injection of BYFAVO given over a 1-minute period [see Clinical Pharmacology (12.2)].
Titrate subsequent doses of BYFAVO on the basis of clinical judgment and assessment of the depth of sedation. If maintenance of procedural sedation is inadequate, consider alternative medications [see Clinical Studies (14)].
2.2 Basic Dosing Information
- Individualize BYFAVO dosing and titrate to desired clinical response.
- In clinical studies, fentanyl 25 to 75 mcg was administered for analgesia prior to the first dose of BYFAVO. Supplemental doses of fentanyl were administered as needed for analgesia [see Clinical Studies (14)].
- Recommended dosing guidelines:
- *
- ASA = American Society of Anesthesiologists Physical Status Classification System
Induction of Procedural Sedation For adult patients: Administer 5 mg intravenously over a 1-minute time period. For ASA* III and IV patients: Administer 2.5 mg to 5 mg intravenously over 1 minute based on the general condition of the patient. Maintenance of Procedural Sedation (as needed) For adult patients: Administer 2.5 mg intravenously over 15 seconds.
At least 2 minutes must elapse prior to administration of any supplemental dose.For ASA III and IV patients: Administer 1.25 mg to 2.5 mg intravenously over 15 seconds.
At least 2 minutes must elapse prior to administration of any supplemental dose.2.3 Preparation
Reconstitution of BYFAVO (remimazolam) for injection
- Strict aseptic technique must be maintained during handling of BYFAVO.
- This product does not contain preservative.
- Once removed from packaging, protect vials from light.
- Each single-patient-use vial contains 20 mg BYFAVO lyophilized powder for reconstitution. The product must be prepared immediately before use.
- To reconstitute, add 8.2 mL sterile 0.9% Sodium Chloride Injection, USP, to the vial, directing the stream of solution toward the wall of the vial. Gently swirl the vial (do not shake) until the contents are fully dissolved. The reconstituted product will deliver a final concentration of 2.5 mg/mL solution of BYFAVO.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Upon reconstitution, the solution should be a clear, colorless to pale yellow solution. Discard if particulate matter or discoloration is observed.
- If not used immediately, reconstituted BYFAVO may be stored in the vial for up to 8 hours under controlled room temperature at 20°C to 25°C (68°F to 77°F). After 8 hours, any unused portion must be discarded.
2.4 Administration with Other Fluids
-
BYFAVO has been shown to be compatible with the following intravenous fluids:
- 0.9% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- 20% Dextrose Injection, USP
- 5% Dextrose and 0.45% Sodium Chloride Injection, USP.
- BYFAVO has also been shown to be compatible with Ringer's Solution, a solution containing Sodium Chloride, Potassium Chloride and Calcium Chloride Dihydrate.
- BYFAVO has been shown to be incompatible with the following intravenous fluids:
- Lactated Ringer's Solution, a solution containing Sodium Chloride, Sodium Lactate, Potassium Chloride, and Calcium Chloride Dihydrate. Lactated Ringer's Solution is also known as Ringer's Lactate Solution, Compound Sodium Lactate Solution, and Hartmann's Solution.
- Acetated Ringer's Solution, a solution containing Sodium Chloride, Sodium Acetate, Potassium Chloride, and Calcium Chloride Dihydrate.
- BYFAVO compatibility with other agents has not been adequately evaluated.
- Do not mix BYFAVO with other drugs or fluids prior to administration.
- 3 DOSAGE FORM AND STRENGTHS
-
4 CONTRAINDICATIONS
BYFAVO is contraindicated in patients with a history of severe hypersensitivity reaction to dextran 40 or products containing dextran 40 [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Personnel and Equipment for Monitoring and Resuscitation
Clinically notable hypoxia, bradycardia, and hypotension were observed in Phase 3 studies of BYFAVO. Continuously monitor vital signs during sedation and through the recovery period.
Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO.
Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation.
Resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO [see Dosage and Administration (2.1)].
Consider the potential for worsened cardiorespiratory depression prior to using BYFAVO concomitantly with other drugs that have the same potential (e.g., opioid analgesics or other sedative-hypnotics) [see Drug Interactions (7.1)].
Administer supplemental oxygen to sedated patients through the recovery period.
A benzodiazepine reversal agent (flumazenil) should be immediately available during administration of BYFAVO [see Overdosage (10)].
5.2 Risks from Concomitant Use with Opioid Analgesics and Other Sedative-Hypnotics
Concomitant use of benzodiazepines, including BYFAVO, and opioid analgesics may result in profound sedation, respiratory depression, coma, and death [see Drug Interactions (7.1)].
The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including other benzodiazepines and propofol.
Titrate the dose of BYFAVO when administered with opioid analgesics and sedative-hypnotics to the desired clinical response.
Continuously monitor sedated patients for hypotension, airway obstruction, hypoventilation, apnea, and oxygen desaturation. These cardiorespiratory effects may be more likely to occur in patients with obstructive sleep apnea, the elderly, and ASA III or IV patients.
5.3 Hypersensitivity Reactions
BYFAVO contains dextran 40, which can cause hypersensitivity reactions, including rash, urticaria, pruritus, and anaphylaxis. BYFAVO is contraindicated in patients with a history of severe hypersensitivity reaction to dextran 40 or products containing dextran 40 [see Contraindications (4), Adverse Reactions (6)].
5.4 Neonatal Sedation and Withdrawal Syndrome
Receiving benzodiazepines late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate. Monitor neonates exposed to benzodiazepines, including BYFAVO, during pregnancy or labor for signs of sedation and monitor neonates exposed to benzodiazepines during pregnancy for signs of withdrawal and manage these neonates accordingly [see Use in Specific Populations (8.1)].
5.5 Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours.
The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans [see Use in Specific Populations (8.1, 8.4), Nonclinical Pharmacology (13.2)].
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections:
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BYFAVO was evaluated in three prospective, randomized, double-blind, multicenter, parallel group clinical studies in 630 patients undergoing colonoscopy (two studies) or bronchoscopy (one study). Colonoscopy Study 1 and the bronchoscopy study evaluated American Society of Anesthesiologists (ASA) physical status I to III patients, and Colonoscopy Study 2 evaluated ASA III and IV patients.
All three studies evaluated the safety of BYFAVO compared to placebo with midazolam rescue and an open-label midazolam treatment arm. Patients were administered a total dose ranging from 5 to 30 mg of BYFAVO. In these studies, the most common adverse reactions (incidence greater than 10%) following BYFAVO administration were hypotension, hypertension, diastolic hypertension, systolic hypertension, hypoxia, and diastolic hypotension. There were two patients who experienced an adverse reaction that led to discontinuation of study drug. One patient in the BYFAVO arm in the bronchoscopy study discontinued treatment due to bradycardia, hypertension, hypotension, hypoxia, and respiratory rate increase. One patient in the open-label midazolam arm in Colonoscopy Study 2 discontinued due to respiratory acidosis. No deaths were reported during the studies.
Tables 1-3 provide a summary of the common adverse reactions observed in each of the three Phase 3 studies with BYFAVO.
Table 1: Common Adverse Reactions in Colonoscopy Study 1 (Incidence >2%), ASA I to III Adverse Reaction BYFAVO
N = 296Placebo (with Midazolam Rescue*)
N = 60Midazolam
N = 102n (%) n (%) n (%) - *
- 57/60 (95%) patients received midazolam rescue.
- †
- Hypotension defined as a fall in systolic BP to ≤80 mmHg or in diastolic BP to ≤40 mmHg, or a fall in systolic or diastolic BP of 20% or more below baseline or necessitating medical intervention.
- ‡
- Hypertension defined as an increase in systolic BP to ≥180 mmHg or in diastolic BP to ≥100 mmHg, or an increase of systolic or diastolic BP of 20% or more over baseline or necessitating medical intervention.
Hypotension† 115 (39%) 25 (42%) 63 (62%) Hypertension‡ 59 (20%) 17 (28%) 18 (18%) Bradycardia 33 (11%) 7 (12%) 16 (16%) Diastolic hypertension‡ 29 (10%) 6 (10%) 9 (9%) Tachycardia 23 (8%) 7 (12%) 13 (13%) Diastolic hypotension† 23 (8%) 4 (7%) 9 (9%) Systolic hypertension‡ 16 (5%) 5 (8%) 6 (6%) Table 2: Common Adverse Reactions in Bronchoscopy Study (Incidence >2%) Adverse Reaction BYFAVO
N = 303Placebo (with Midazolam Rescue*)
N = 59Midazolam
N = 69n (%) n (%) n (%) - *
- 57/59 (97%) patients received midazolam rescue.
- †
- Hypotension defined as a fall in systolic BP to ≤80 mmHg or in diastolic BP to ≤40 mmHg, or a fall in systolic or diastolic BP of 20% or more below baseline or necessitating medical intervention.
- ‡
- Hypertension defined as an increase in systolic BP to ≥180 mmHg or in diastolic BP to ≥100 mmHg, or an increase of systolic or diastolic BP of 20% or more over baseline or necessitating medical intervention.
Hypotension† 99 (33%) 28 (47%) 23 (33%) Hypertension‡ 85 (28%) 9 (15%) 19 (28%) Diastolic hypertension‡ 77 (25%) 15 (25%) 16 (23%) Systolic hypertension‡ 67 (22%) 13 (22%) 17 (25%) Hypoxia 66 (22%) 12 (20%) 13 (19%) Respiratory rate increased 43 (14%) 6 (10%) 10 (14%) Diastolic hypotension† 41 (14%) 17 (29%) 16 (23%) Nausea 12 (4%) 2 (3%) 2 (3%) Bradycardia 11 (4%) 4 (7%) 4 (6%) Pyrexia 11 (4%) 1 (2%) 1 (1%) Headache 8 (3%) 0 (0%) 3 (4%) Table 3: Common Adverse Reactions in Colonoscopy Study 2 (Incidence >2%), ASA III and IV Adverse Reaction BYFAVO
N = 31Placebo (with Midazolam Rescue*)
N = 16Midazolam
N = 30n (%) n (%) n (%) - *
- 16/16 (100%) patients received midazolam rescue.
- †
- Hypotension defined as a fall in systolic BP to ≤80 mmHg or in diastolic BP to ≤40 mmHg, or a fall in systolic or diastolic BP of 20% or more below baseline or necessitating medical intervention.
- ‡
- Hypertension defined as an increase in systolic BP to ≥180 mmHg or in diastolic BP to ≥100 mmHg, or an increase of systolic or diastolic BP of 20% or more over baseline or necessitating medical intervention.
Hypotension† 18 (58%) 11 (69%) 17 (57%) Hypertension‡ 13 (42%) 6 (38%) 13 (43%) Respiratory acidosis 6 (19%) 2 (13%) 8 (27%) Diastolic hypertension‡ 3 (10%) 0 (0%) 0 (0%) Systolic hypertension‡ 2 (6%) 0 (0%) 0 (0%) Bradycardia 1 (3%) 1 (6%) 4 (13%) Respiratory rate decreased 1 (3%) 1 (6%) 2 (7%) Diastolic hypotension† 1 (3%) 1 (6%) 0 (0%) Blood pressure diastolic increased 1 (3%) 0 (0%) 0 (0%) Blood pressure increased 1 (3%) 0 (0%) 0 (0%) Blood pressure systolic increased 1 (3%) 0 (0%) 0 (0%) Upper respiratory tract infection 1 (3%) 0 (0%) 0 (0%) Adverse reaction data from Colonoscopy Study 1 and the bronchoscopy study analyzed according to the cumulative dose of concomitant fentanyl (<100 mcg, 100-150 mcg and >150 mcg) suggest an increase in some adverse reactions with increasing fentanyl dose, such as hypotension, hypertension, bradycardia, hypoxia, and increased respiratory rate (see Table 4 and Table 5). There were too few patients in each fentanyl stratum in Colonoscopy Study 2 to perform this analysis.
Table 4: Common Adverse Reactions* in Colonoscopy Study 1 by Cumulative Fentanyl Dose BYFAVO Placebo (with Midazolam Rescue†) Midazolam Fentanyl dose (mcg) <100 100-150 >150 <100 100-150 >150 <100 100-150 >150 N = 148 N = 146 N = 2 N = 9 N = 43 N = 8 N = 31 N = 62 N = 9 Adverse Reaction n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) - *
- Incidence >2% of patients.
- †
- 57/60 (95%) patients received midazolam rescue.
- ‡
- Hypotension defined as a fall in systolic BP to ≤80 mmHg or in diastolic BP to ≤40 mmHg, or a fall in systolic or diastolic BP of 20% or more below baseline or necessitating medical intervention.
- §
- Hypertension defined as an increase in systolic BP to ≥180 mmHg or in diastolic BP to ≥100 mmHg, or an increase of systolic or diastolic BP of 20% or more over baseline or necessitating medical intervention.
Hypotension‡ 49
(33%)64
(44%)2
(100%)5
(56%)17
(40%)3
(38%)18
(58%)36
(58%)9
(100%)Hypertension§ 24
(16%)35
(24%)0
(0%)1
(11%)14
(33%)2
(25%)3
(10%)12
(19%)3
(33%)Bradycardia 12
(8%)20
(14%)1
(50%)0
(0%)5
(12%)2
(25%)1
(3%)13
(21%)2
(22%)Diastolic hypertension§ 9
(6%)20
(14%)0
(0%)0
(0%)3
(7%)3
(38%)2
(6%)7
(11%)0
(0%)Tachycardia 10
(7%)12
(8%)1
(50%)0
(0%)6
(14%)1
(13%)2
(6%)8
(13%)3
(33%)Diastolic hypotension‡ 10
(7%)13
(9%)0
(0%)0
(0%)3
(7%)1
(13%)3
(10%)4
(6%)2
(22%)Systolic hypertension§ 5
(3%)11
(8%)0
(0%)0
(0%)3
(7%)2
(25%)4
(13%)2
(3%)0
(0%)Table 5: Common Adverse Reactions* in Bronchoscopy Study by Cumulative Fentanyl Dose BYFAVO Placebo (with Midazolam Rescue†) Midazolam Fentanyl dose (mcg) <100 100-150 >150 <100 100-150 >150 <100 100-150 >150 N = 215 N = 63 N = 25 N = 26 N = 18 N = 15 N = 29 N = 27 N = 13 Adverse Reaction n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) - *
- Incidence >2% of patients.
- †
- 57/59 (97%) patients received midazolam rescue.
- ‡
- Hypotension defined as a fall in systolic BP to ≤ 80 mmHg or in diastolic BP to ≤40 mmHg, or a fall in systolic or diastolic BP of 20% or more below baseline or necessitating medical intervention.
- §
- Hypertension defined as an increase in systolic BP to ≥180 mmHg or in diastolic BP to ≥100 mmHg, or an increase of systolic or diastolic BP of 20% or more over baseline or necessitating medical intervention.
Hypotension‡ 52
(24%)32
(51%)16
(64%)7
(27%)9
(50%)12
(80%)7
(24%)7
(26%)9
(69%)Hypertension§ 43
(20%)25
(40%)18
(72%)2
(8%)2
(11%)5
(33%)3
(10%)8
(30%)8
(62%)Diastolic hypertension§ 65
(30%)12
(19%)0
(0%)11
(42%)3
(17%)1
(7%)10
(34%)6
(22%)0
(0%)Systolic hypertension§ 55
(26%)11
(17%)1
(4%)10
(38%)3
(17%)0
(0%)9
(31%)6
(22%)2
(15%)Hypoxia 35
(16%)22
(35%)9
(36%)6
(23%)2
(11%)4
(27%)2
(7%)5
(19%)6
(46%)Respiratory rate increased 22
(10%)12
(19%)9
(36%)1
(4%)2
(11%)3
(20%)2
(7%)5
(19%)3
(23%)Diastolic hypotension‡ 28
(13%)13
(21%)0
(0%)8
(31%)7
(39%)2
(13%)7
(24%)6
(22%)3
(23%)Nausea 9
(4%)1
(2%)2
(8%)0
(0%)0
(0%)2
(13%)1
(3%)1
(4%)0
(0%)Bradycardia 3
(1%)4
(6%)4
(16%)2
(8%)1
(6%)1
(7%)0
(0%)2
(7%)2
(15%)Pyrexia 7
(3%)2
(3%)2
(8%)0
(0%)0
(0%)1
(7%)1
(3%)0
(0%)0
(0%)Headache 5
(2%)2
(3%)1
(4%)0
(0%)0
(0%)0
(0%)0
(0%)3
(11%)0
(0)% -
7 DRUG INTERACTIONS
7.1 Opioid Analgesics and Other Sedative-Hypnotics
The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including opioid analgesics, other benzodiazepines, and propofol. Continuously monitor vital signs during sedation and through the recovery period. Titrate the dose of BYFAVO when administered with opioid analgesics and sedative-hypnotics to the desired clinical response [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [see Warnings and Precautions (5.4), Clinical Considerations]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
In animal studies, reduced fetal weights but no evidence of malformations or embryofetal lethality were noted in a study in which pregnant rabbits were treated intravenously with 4 times the maximum recommended human dose (MRHD) of 30 mg during organogenesis. Adequate rodent reproductive and developmental toxicology studies have not been completed to fully evaluate the effects of BYFAVO.
Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to BYFAVO during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to BYFAVO during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.4)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco, and other medications, have not confirmed these findings.
Animal Data
Reduced fetal weights but no evidence of malformation or embryofetal lethality were noted in a study in which pregnant rabbits were treated intravenously with 5 mg/kg remimazolam (approximately 4 times the MRHD of 30 mg/day based on AUC) from Gestation Day 6 to 20 in the presence of maternal toxicity (reduced food intake and body weights).
In a study that did not test exposures comparable to the MRHD of 30 mg/day over the full period of organogenesis, there was an increase in early resorptions (embryolethality) but no evidence of malformations when female rats were treated from Gestation Day 6 through 17 with up to 30 mg/kg remimazolam via intravenous bolus (approximately 0.3 times the MRHD based on AUC by the end of the dosing interval) in the presence of maternal toxicity (convulsion in one mid dose and one high dose dam).
In a pre- and postnatal development study that did not test exposures comparable to the MRHD of 30 mg/day over the full treatment period, there were no adverse effects on survival or development of offspring when pregnant rats were treated with up to 30 mg/kg remimazolam (<0.3 times the MRHD by the end of the gestational period) by intravenous bolus injection from Gestation Day 6 through Lactation Day 20 with minimal evidence of maternal toxicity (sedation).
No evidence of adverse effects on physical development, a functional observational battery of behavioral assessments, or fertility were noted in pups born to pregnant rabbits that were treated by intravenous infusion of up to 20 mg/kg/day remimazolam (approximately 19 times the MRHD based on AUC) from 14 days prior to mating until Lactation Day 30 despite the presence of maternal toxicity (sedation, convulsions, and mortality). Learning and memory of the first-generation offspring was not evaluated in this study.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits [see Warnings and Precautions (5.4, 5.5), Use in Specific Populations (8.4), Nonclinical Toxicology (13.2)].
8.2 Lactation
Risk Summary
There are reports of sedation, poor feeding, and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data on the effects of remimazolam in human milk, the effects on the breastfed infant or the effects on milk production. Remimazolam is present in animal milk (see Data). When a drug is present in animal milk, it is likely that it will be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BYFAVO and any potential adverse effects on the breastfed child from BYFAVO or from the underlying maternal condition.
Clinical Considerations
Infants exposed to BYFAVO through breast milk should be monitored for sedation, poor feeding, and poor weight gain. A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk during treatment and for 5 hours (approximately 5 elimination half-lives) after BYFAVO administration in order to minimize drug exposure to a breastfed infant.
Data
In rabbits administered daily intravenous infusions of remimazolam at 12.5 and 20 mg/kg/day from 14 days before mating until Lactation Day 30, remimazolam and the metabolite CNS7054 were present in milk samples obtained after the end of an infusion on Day 10 or 11 of lactation. Remimazolam was not quantifiable in plasma samples obtained from rabbit kits taken in the morning on Day 10 or 11 of lactation. However, metabolite CNS7054 was present at low levels in 2 of the 5 kits sampled.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. No studies are available in any pediatric population and extrapolation of adult effectiveness data to the pediatric population is not possible.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as BYFAVO, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss; however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data [see Warnings and Precautions (5.4), Warnings and Precautions (5.5), Use in Specific Populations (8.1), Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
Of the total number of subjects treated with BYFAVO in clinical studies for procedural sedation, there were 649 subjects <65 years of age, 221 subjects >65 years of age, 171 subjects between 65-74 years of age, and 50 subjects >75 years of age.
No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. Some data suggest a potential of greater sensitivity (a faster onset of loss of consciousness and a longer duration of sedation) of some older individuals.
Administer supplemental doses of BYFAVO slowly to achieve the level of sedation required for the procedure, and monitor all patients for cardiorespiratory complications.
8.6 Hepatic Impairment
In patients with severe hepatic impairment, the dose of BYFAVO should be carefully titrated to effect. Depending on the overall status of the patient, lower frequency of supplemental doses may be needed to achieve the level of sedation required for the procedure. All patients should be monitored for sedation-related cardiorespiratory complications [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
BYFAVO contains the benzodiazepine, remimazolam. Benzodiazepines are a class of sedative drugs with a known potential for abuse. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. In a human abuse potential study conducted in recreational sedative abusers (n = 39), remimazolam (5 and 10 mg, IV) produced responses on positive subjective measures such as "Drug Liking," "Overall Drug Liking," "Take Drug Again," and "Good Drug Effects" that were statistically similar to those produced by the sedative midazolam (2.5 and 5 mg), and statistically greater than the responses on these measures that were produced by placebo.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. In a monkey physical dependence study, chronic administration of remimazolam produced withdrawal signs such as tremors, muscle rigidity, restlessness, impaired motor activity, and a reduction in food consumption upon drug discontinuation. One monkey of six in this study exhibited systemic convulsions and dissociation from the environment. These behaviors are consistent with benzodiazepine withdrawal, which suggests that remimazolam produces physical dependence.
-
10 OVERDOSAGE
Clinical Presentation
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
Management of Overdosage
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help Line (1-800-222-1222), or medical toxicologist for additional overdosage management recommendations.
-
11 DESCRIPTION
Each glass, single-patient-use, sterile vial of BYFAVO (remimazolam) for injection contains 20 mg remimazolam, equivalent to 27.2 mg remimazolam besylate.
Remimazolam is a benzodiazepine. Its chemical description is 4H-imidazol[1,2-a][1,4]benzodiazepine-4-propionic acid, 8-bromo-1-methyl-6-(2-pyridinyl)-(4S)-, methyl ester, benzenesulfonate (1:1). The structural formulas are shown below.
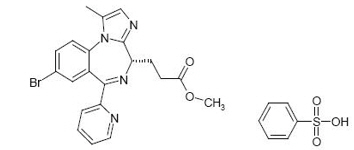
Molecular weight of BYFAVO (free base): 439.3 g/mol.
Molecular weight of BYFAVO besylate: 597.5 g/mol.
BYFAVO besylate powder is sparingly soluble in water.
BYFAVO 20 mg contains: 82 mg dextran 40 and 55 mg lactose monohydrate as bulking agents/stabilizers. The pH is adjusted with hydrochloride/sodium hydroxide. Upon reconstitution with saline, BYFAVO has a pH of 2.9 to 3.9.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
BYFAVO is a benzodiazepine. BYFAVO binds to brain benzodiazepine sites (gamma amino butyric acid type A [GABAA] receptors), while its carboxylic acid metabolite (CNS7054) has a 300 times lower affinity for the receptor. BYFAVO, like other benzodiazepines, did not show clear selectivity between subtypes of the GABAA receptor.
12.2 Pharmacodynamics
Dose finding studies determined the IV dosing recommendation of the initial 5 mg bolus, followed by 2.5 mg top-up doses. Median time to peak sedation, defined as the lowest Modified Observer's Assessment of Alertness/Sedation (MOAA/S) score after the initial dose, in the Phase 3 trials was 3 to 3.5 minutes and median time to fully alert, defined as time to the first of three consecutive MOAA/S scores of five, following the last dose of BYFAVO was 11 to 14 minutes.
Cardiac Electrophysiology
In a thorough QT study, 57 healthy volunteers were given an IV push of 10 mg or 20 mg BYFAVO, intravenous midazolam (2.5 mg or 7.5 mg) or placebo, or a single tablet of moxifloxacin 400 mg given orally. The largest mean placebo-adjusted change-from-baseline QTc (upper bound of 2-sided 90% confidence interval) was 6.7 (9.5) ms, 10.7 (13.4) ms, 4.5 (7.3) ms, and 8.1 (10.8) ms, respectively, after treatment with 10 mg or 20 mg BYFAVO, or 2.5 mg or 7.5 mg midazolam.
BYFAVO treatment is associated with increases in heart rate. The largest mean placebo-adjusted change-from-baseline HR (upper bound of 2-sided 90% confidence interval) was 12.3 (14.2) bpm and 15.2 (17.1) bpm, respectively, after treatment with 10 mg and 20 mg BYFAVO.
12.3 Pharmacokinetics
- BYFAVO has a terminal elimination half-life from plasma of 37 to 53 minutes.
- Mean distribution half-life (t1/2α) is between 0.5 and 2 minutes.
- Half-life (t1/2) is prolonged with increasing severity of hepatic impairment leading to a need for careful dose titration in patients with severe hepatic impairment.
- Clearance (54 to 75 L/h) is not related to body weight.
- In healthy subjects at least 80% and in colonoscopy patients 50% to 60% of dose is excreted in urine as inactive metabolite.
Absorption
BYFAVO is administered intravenously. BYFAVO overall maximum plasma concentration (Cmax) after IV administration of 0.01 to 0.5 mg/kg was 189 to 6,960 ng/mL, and overall area under the concentration versus time curve from time 0 to infinity (AUC0-∞) was 12.1 to 452 ng∙h/mL; BYFAVO cumulative dose versus BYFAVO total exposure (AUC0-∞) suggested a close to dose-proportional relationship. Metabolite Cmax was achieved approximately 20-30 minutes post dose. Metabolite AUC0-∞ was 231 to 7,090 ng∙h/mL.
Distribution
BYFAVO volume of distribution (Vz) was 0.76 to 0.98 L/kg. Plasma protein binding of BYFAVO was >91%, primarily to human serum albumin.
Elimination
BYFAVO has a terminal elimination half-life from plasma of 37 to 53 minutes and mean distribution half-life (t1/2α) is between 0.5 and 2 minutes.
Metabolism
The main route of metabolism of BYFAVO is via conversion to primary inactive metabolite CNS7054, which is then subject to hydroxylation and glucuronidation. Conversion to CNS7054 is mediated by tissue carboxylesterases (primarily type 1A), with no meaningful contribution by cytochrome P450 enzymes. The t1/2 of the metabolite was 2.4 to 3.8 hours.
Specific Populations
Patients with Renal Impairment
The pharmacokinetics of BYFAVO were not altered in patients with mild to end stage renal disease not requiring dialysis. In a renal impairment study, BYFAVO PK parameters (e.g., AUC and Cmax) were not statistically different in subjects with varying degrees of renal function (from normal to severely impaired). Increased exposure to inactive metabolite CNS7054 was observed with increasing degree of renal impairment.
Patients with Hepatic Impairment
A Phase 1 open-label, single-dose trial evaluated the PK and safety of BYFAVO given as an IV bolus of 0.1 mg/kg over 1 minute in subjects with hepatic impairment (8 moderately hepatically impaired subjects and 3 severely hepatically impaired subjects) and 9 matched healthy subjects.
The Cmax values of total BYFAVO were 10% to 20% lower in subjects with hepatic impairment than in healthy subjects. Larger Vz (33% increase in moderately impaired and 41% increase in severely impaired) and Vss (50% increase in moderately impaired and 115% increase in severely impaired), and prolonged t1/2 (60 minutes in moderately impaired and 105 minutes in severely impaired as compared to 42 minutes in healthy subjects), of BYFAVO were observed with increasing severity of hepatic impairment. Sedation lasted longer and recovery took longer for subjects with hepatic impairment compared to healthy subjects. The average duration of loss of consciousness and recovery time was 3.2 minutes and 12.1 minutes, respectively for subjects in the moderately hepatically impaired group. These times were 2.0 minutes and 16.7 minutes, respectively, for the subjects in the severely hepatically impaired group. Healthy control subjects had a loss of consciousness of 1.6 minutes and a recovery time of 8.0 minutes.
In patients with severe hepatic impairment, the dose of BYFAVO should be carefully titrated to effect. Depending on the overall status of the patient, less frequency of supplemental doses may be needed to achieve the level of sedation required for the procedure. All patients should be monitored for sedation-related cardiorespiratory complications.
Drug Interactions
BYFAVO and the metabolite CNS7054 caused no relevant inhibition of cytochrome P450 isoenzymes 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4. There were no inducing effects on CYP1A2, 2B6, and 3A4. BYFAVO was not a relevant substrate of a panel of human drug transporters (OATP1B1, OATP1B3, BCRP).
No relevant inhibition of human drug transporters (OAT3, OCT2, OATP1B1, OATP1B3, OAT1, BCRP) was seen with BYFAVO or CNS7054. Remifentanil did not influence the hydrolysis of BYFAVO by human liver S9 fractions, reducing the possibility of an interaction by competition for liver carboxylesterases.
These results together show a very low potential of BYFAVO for pharmacokinetic drug interactions.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been performed to evaluate the carcinogenic potential of remimazolam.
Mutagenesis
Remimazolam was not mutagenic or clastogenic when evaluated in an in vitro bacterial reverse mutation assay (Ames test), an in vivo rat micronucleus assay, mouse lymphoma cells, in vivo rat bone marrow micronucleus assay, or comet assay.
Impairment of Fertility
In a study that did not test exposures comparable to the MRHD of 30 mg/day, there were no adverse effects on male or female fertility when male rats were treated for 28 days prior to mating and female rats were treated for 14 days prior to mating with up to 30 mg/kg remimazolam via intravenous bolus (approximately 0.03 times the MRHD based on AUC).
There was no impact on female fertility when female rabbits were administered remimazolam by intravenous infusion (up to 4 hours/day) up to 20 mg/kg/day (approximately 17 times the MRHD of 30 mg/day based on AUC) from 14 days prior to mating.
No adverse effects on histology of the testes and epididymides or evaluation of spermatid count, sperm motility, and sperm morphology were reported in a repeat-dose toxicity study in which male minipigs were administered remimazolam by intravenous infusion (6 hours) up to 120 mg/kg/day (approximately 400 times the MRHD based on AUC) for 28 days followed by a 14-day recovery period.
13.2 Animal Toxicology and/or Pharmacology
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss; however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data [See Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.4)].
-
14 CLINICAL STUDIES
The safety and efficacy of BYFAVO compared to a saline placebo with midazolam rescue treatment group and an open-label midazolam treatment group was evaluated in three randomized, double-blind, multicenter Phase 3 studies conducted in 969 adult patients receiving procedural sedation.
14.1 Colonoscopy Study 1 (NCT 02290873)
This Phase 3 study was conducted in 461 ASA I to III patients undergoing colonoscopy. BYFAVO 5 mg (2 mL) IV was administered as an initial bolus, followed by 2.5 mg (1 mL) top-up doses versus placebo 2 mL administered as an initial bolus, followed by 1 mL top-up doses. Midazolam rescue was dosed per investigator discretion in both treatment groups. Fentanyl was administered as an analgesic pre-treatment at an initial dose of 50 to 75 mcg IV (or a reduced dose for ASA III patients) immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate or a maximum dose of 200 mcg had been administered. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of 1 to 5 L/minute until the patient was fully alert after procedure completion. Colonoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3. The primary efficacy endpoint for BYFAVO versus placebo was success of the colonoscopy procedure, defined as a composite of the following:
- Completion of the colonoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
There were 63 patients (13.8%) who were aged 65 years or older, 218 patients (47.6%) who were male, 339 (74.0%) who were white, 80 (17.5%) who were Black or African American, 31 (6.8%) who were Asian, and 73 (15.9%) who were Hispanic or Latino. There were 143 patients in ASA I, 285 in ASA II, and 30 in ASA III. As shown in Table 6, the colonoscopy sedation success rate was statistically significantly higher in the BYFAVO group than in the placebo group.
Table 6. Colonoscopy Sedation Success Rate – Colonoscopy Study 1 Cohort Sedation Success Rate
n/N (%)n/N = number of successes/number of subjects in group. Remimazolam 272/298 (91.3%) Placebo 1/60 (1.7%) The reasons for procedural sedation failure are shown in Table 7.
Table 7. Reasons for Procedural Sedation Failure – Colonoscopy Study 1 Reason Remimazolam
N = 298
n (%)Placebo
N = 60
n (%)Rescue sedative medication taken 10 (3.4%) 57 (95%) Too many doses within the predefined time window 18 (6.0%) 44 (73.3%) Procedure not completed 7 (2.3%) 1 (1.7%) Table 8 shows the number of top-up doses required, and the total doses of study medication, fentanyl, and rescue medication administered.
Table 8. Number of Top-up Doses and Total Doses of Study Medication, Fentanyl, and Rescue Medication – Colonoscopy Study 1 Number of Top-up Doses of Study Drug
(Mean ± SD)Total Amount of Study Drug (mg)
(Mean ± SD)Total Amount of Fentanyl (mcg)
(Mean ± SD)Total Amount of Midazolam Rescue Medication (mg)
(Mean ± SD)Remimazolam 2.2 ± 1.6 10.5 ± 4.0 88.9 ± 21.7 0.3 ± 2.1 Placebo 5.1 ± 0.5 0 121.3 ± 34.4 6.8 ± 4.2 Summaries of the time to start procedure, duration of procedure, time to fully alert, and time to ready for discharge are shown in Table 9.
Table 9. Time to Start Procedure, Duration of Procedure, Time to Fully Alert, and Time to Ready for Discharge for the Remimazolam Cohort – Colonoscopy Study 1 Time to start procedure (minutes)* Median (95% confidence interval) 4.0 (4.0, 4.0) Min, Max 0, 26 Duration of procedure (minutes)† Median (95% confidence interval) 12.0 (11.0, 13.0) Min, Max 3, 33 Number (proportion) of procedures lasting longer than 30 minutes 1/291 (0.3%) Time to fully alert after end of colonoscopy (minutes)† Median (95% confidence interval) 6.0 (5.0, 7.0) Min, Max 0, 44 Time to ready to discharge after end of colonoscopy (minutes)† Median (95% confidence interval) 44.0 (42.0, 46.0) Min, Max 3, 79 14.2 Bronchoscopy Study (NCT 02296892)
This Phase 3 study was conducted in 431 ASA I to III patients undergoing bronchoscopy. BYFAVO 5 mg (2 mL) IV was administered as an initial bolus, followed by 2.5 mg (1 mL) top-up doses versus placebo 2 mL administered as an initial bolus, followed by 1 mL top-up doses. Midazolam rescue was dosed per investigator discretion in both treatment groups. Fentanyl was administered as an analgesic pre-treatment at an initial dose of 25 to 50 mcg IV immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate. A maximum dose of fentanyl 200 mcg was recommended. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of 1 to 15 L/minute until the patient was fully alert after procedure completion. Bronchoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3. The primary efficacy endpoint for BYFAVO versus placebo was successful sedation for the bronchoscopy procedure, defined as a composite of the following:
- Completion of the bronchoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
There were 209 patients (48.5%) who were 65 years or older, 198 patients (45.9%) who were male, 358 (83.1%) who were white, 62 (14.4%) who were Black or African American, 5 (1.2%) who were Asian, and 8 (1.9%) who were Hispanic or Latino. There were 15 patients in ASA I, 254 in ASA II, and 162 in ASA III. As shown in Table 10, the bronchoscopy sedation success rate was statistically significantly higher for the BYFAVO group than for the placebo group.
Table 10. Bronchoscopy Success Rates Cohort Total Success Rate
n/N (%)n/N = number of successes/number of subjects in group. Remimazolam 250/310 (80.6%) Placebo 3/63 (4.8%) The reasons for procedural sedation failure are shown in Table 11.
Table 11. Reasons for Procedural Sedation Failure – Bronchoscopy Study Reason Remimazolam
N = 310
n (%)Placebo
N = 63
n (%)Rescue sedative medication taken 49 (15.8%) 57 (90.5%) Too many doses within the predefined time window 14 (4.5%) 10 (15.9%) Procedure not completed 9 (2.9%) 3 (4.8%) Table 12 shows the number of top-up doses required, and the total doses of study medication, fentanyl, and rescue medication administered.
Table 12. Number of Top-up Doses and Total Doses of Study Medication, Fentanyl, and Rescue Medication – Bronchoscopy Study Number of Top-up Doses of Study Drug
(Mean ± SD)Total Amount of Study Drug (mg)
(Mean ± SD)Total Amount of Fentanyl (mcg)
(Mean ± SD)Total Amount of Midazolam Rescue Medication (mg)
(Mean ± SD)Remimazolam 2.6 ± 2.0 11.5 ± 5.1 81.8 ± 54.3 1.3 ± 3.5 Placebo 4.1 ± 0.8 0 118.8 ± 79.1 5.9 ± 3.7 Summaries of the time to start procedure, duration of procedure, time to fully alert, and time to ready for discharge are shown in Table 13.
Table 13. Time to Start Procedure, Duration of Procedure, Time to Fully Alert and Time to Ready for Discharge for the Remimazolam Cohort – Bronchoscopy Study Time to start procedure (minutes)* Median (95% confidence interval) 4.1 (4.0, 4.8) Min, Max 1,41 Duration of procedure (minutes)† Median (95% confidence interval) 10.0 (8.0, 11.0) Min, Max 1, 68 Number (proportion) of procedures lasting longer than 30 minutes† 28/299 (9.4%) Time to fully alert after end of bronchoscopy (minutes)† Median (95% confidence interval) 6.0 (5.2, 7.1) Min, Max 1.1, 107 Time to ready to discharge after end of bronchoscopy (minutes)† Median (95% confidence interval) 60.0 (57.0, 63.0) Min, Max 6.6, 284 14.3 Colonoscopy Study 2 (NCT 02532647)
This Phase 3 study was conducted in 77 ASA III and IV patients undergoing colonoscopy. BYFAVO 2.5 mg (1 mL) to 5 mg (2 mL) IV was administered as an initial bolus, followed by 1.25 mg (0.5 mL) to 2.5 mg (1 mL) top-up doses versus placebo 1 to 2 mL administered with midazolam rescue, dosed per investigator discretion. Fentanyl was administered as an analgesic pre-treatment at an initial maximum dose of 50 mcg (with dose reduction for debilitated patients), immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate or a maximum dose of 200 mcg had been administered. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of up to 4 L/minute until the patient was fully alert after procedure completion. Colonoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3.
The primary objective of the study was to assess the safety of multiple doses of BYFAVO compared to placebo and midazolam. Procedure success was a secondary objective and was defined as follows:
- Completion of the colonoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
The total patient population, including all randomized patients who received any amount of study medication, comprised 31 patients in the remimazolam group, 16 patients in the placebo group, and 30 patients in the midazolam group. There were two patients, one each in the remimazolam and midazolam treatment groups, who were randomized, but did not receive a dose of study medication.
There were 31 patients (40.2%) who were aged 65 years or older, 43 patients (55.8%) who were male, 57 (74.0%) who were white, 19 (24.7%) who were Black or African American, 1 (1.30%) who was Asian, and none who were Hispanic or Latino. There were 40 patients in ASA III and 37 patients in ASA IV.
Patients in the remimazolam group received a mean (± SD) of 9.0 (± 3.7) mg of remimazolam and a mean (± SD) of 2.5 (± 10.2) mg of midazolam compared to 7.2 (± 2.5) mg in the placebo group. The mean total dose of fentanyl was lower in the remimazolam group (mean ± SD: 59.7 ± 15.4 mcg) than in the placebo group (mean ± SD: 67.2 ± 21.8 mcg).
In the remimazolam group, 90.3% of patients did not receive any rescue sedative medication, compared to 0.0% in the placebo group.
There were no serious adverse reactions and no discontinuations due to adverse reactions observed in the remimazolam group. The incidence of hypotension (SMQ) was 61.3% in the remimazolam group and 75% in the placebo group.
No inferential statistical tests were performed in this trial. Patients who received BYFAVO for sedation during scheduled colonoscopy responded at a numerically greater rate than patients who received placebo (randomized analysis population – remimazolam: 27/32 [84.4%]; placebo: 0/16 [0%]).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BYFAVO (remimazolam) for injection, for intravenous use is supplied as follows:
NDC 71390-011-11: Carton of 10 × 12 mL vials. Each 12 mL glass vial of BYFAVO (NDC 71390-011-00) provides a sterile lyophilized white to off-white powder intended for single-patient use only and contains 20 mg remimazolam (equivalent to 27.2 mg remimazolam besylate) ready for reconstitution.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F) excursions between 15° and 30°C (59° and 86°F) are allowed.
Reconstituted BYFAVO can be stored in the vial for up to 8 hours under controlled room temperature at 20°C to 25°C (68°F to 77°F).
Protect vials from light once they are removed from packaging.
Discard unused portion.
-
17 PATIENT COUNSELING INFORMATION
Alcohol and Current Medications
Advise patients to notify their healthcare provider about alcohol or medication use. Alcohol and other CNS depressants, such as opioid analgesics and benzodiazepines, can have an additive effect when administered with BYFAVO [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Pregnancy
Advise pregnant females that receiving BYFAVO late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns. Instruct patients to inform their healthcare provider if they are pregnant during treatment with BYFAVO [see Warnings and Precautions (5.4, 5.5), Use in Specific Populations (8.1)].
Effect of Anesthetic and Sedation Drugs on Early Brain Development
Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.4), Nonclinical Toxicology (13.2)].
Lactation
Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients receiving BYFAVO to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs. A lactating woman may consider pumping and discarding breast milk for 5 hours after receiving BYFAVO during procedural sedation to minimize drug exposure to the breastfed infant [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 20 mg Vial Carton
- PRINCIPAL DISPLAY PANEL - NDC: 71390-011-00 - Vial Label
-
INGREDIENTS AND APPEARANCE
BYFAVO
remimazolam besylate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71390-011 Route of Administration INTRAVENOUS DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength remimazolam besylate (UNII: 280XQ6482H) (Remimazolam - UNII:7V4A8U16MB) Remimazolam 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength dextran 40 (UNII: K3R6ZDH4DU) 10 mg in 1 mL lactose monohydrate (UNII: EWQ57Q8I5X) 6.7 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71390-011-11 10 in 1 CARTON 10/06/2020 1 NDC:71390-011-00 8 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212295 10/06/2020 Labeler - Acacia Pharma, Ltd. (779660930) Establishment Name Address ID/FEI Business Operations Cambrex Karlskoga AB 353954043 ANALYSIS(71390-011) , API MANUFACTURE(71390-011) Establishment Name Address ID/FEI Business Operations Cosmo S.p.A. 630431955 ANALYSIS(71390-011) , MANUFACTURE(71390-011) Establishment Name Address ID/FEI Business Operations ITS Testing Services (UK) Ltd. (Intertek Pharmaceutical Services Manchester) 233547491 ANALYSIS(71390-011) Establishment Name Address ID/FEI Business Operations Particle Analytical ApS 306081360 ANALYSIS(71390-011) Establishment Name Address ID/FEI Business Operations Patheon Italia S.p.A. 338336589 ANALYSIS(71390-011) , MANUFACTURE(71390-011) , PACK(71390-011) Establishment Name Address ID/FEI Business Operations Eurofins BioPharma Product Testing Sweden AB 350827847 ANALYSIS(71390-011)