Label: LENMELDY- atidarsagene autotemcel suspension
- NDC Code(s): 83222-0200-1
- Packager: Orchard Therapeutics (Europe) Ltd
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LENMELDY safely and effectively.
See full prescribing information for LENMELDY.
LENMELDY (atidarsagene autotemcel) suspension for intravenous infusion
Initial U.S. Approval 2024INDICATIONS AND USAGE
LENMELDY is an autologous hematopoietic stem cell-based gene therapyindicated for the treatment of children with pre-symptomatic late infantile (PSLI), pre-symptomatic early juvenile (PSEJ) or early symptomatic early juvenile (ESEJ) metachromatic leukodystrophy (MLD). (1)
DOSAGE AND ADMINISTRATION
For autologous use only. For one-time single-dose intravenous use only.
- Children are required to undergo hematopoietic stem cell (HSC) mobilization followed by apheresis to obtain CD34+ cells for LENMELDY manufacturing. ( 2.2)
- Dosing of LENMELDY is based on the number of CD34+ cells in the infusion bag(s) per kg of body weight. ( 2.1)
- The minimum recommended dose is based on the MLD disease subtype. ( 2.1)
- Myeloablative conditioning must be administered before infusion of LENMELDY. ( 2.2)
- Confirm that the child’s identity matches the unique patient identification information on the LENMELDY infusion bag(s) prior to infusion. ( 2.2)
- Do not sample, alter, irradiate, or refreeze LENMELDY. ( 2.2)
- Do not use a leukodepleting filter. ( 2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None ( 4)
WARNINGS AND PRECAUTIONS
-
Thrombosis and Thromboembolic Events: Evaluate the risk factors for thrombosis prior to and after LENMEDLY infusion. Consider prophylaxis with anti-thrombotic agents prior to treatment with LENMELDY. (
5.1)
-
Encephalitis: Monitor children for signs or symptoms of encephalitis after treatment with LENMELDY. (
5.2)
-
Serious Infection: Monitor children for serious infection after myeloablative conditioning and LENMELDY infusion. (
5.3)
- Veno-occlusive Disease: Monitor children for signs and symptoms of VOD including liver function tests in all patients during the first month after LENMELDY infusion. Consider prophylaxis for VOD. ( 5.4)
- Delayed Platelet Engraftment: Monitor children for thrombocytopenia and bleeding until platelet recovery is achieved. ( 5.5)
-
Risk of Neutrophil Engraftment Failure: Monitor absolute neutrophil counts (ANC) after LENMELDY infusion. If neutrophil engraftment does not occur, administer rescue cells. (
5.6)
-
Risk of Insertional Oncogenesis:Monitor children for hematologic malignancies annually after treatment with LENMELDY. (
5.7)
-
Risk of Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (
5.8)
ADVERSE REACTIONS
- The most common non-laboratory adverse reactions (incidence ≥ 10%) were: febrile neutropenia (85%), stomatitis (77%), respiratory tract infections (54%), rash (33%), device related infections (31%), other viral infections (28%), pyrexia (21%), gastroenteritis (21%), and hepatomegaly (18%). (
6.1)
- The most common laboratory abnormalities were: elevated D-dimer (67%), neutropenia (28%), and elevated liver enzymes (23%). (
6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Orchard Therapeutics at toll-free phone 1-888-878-0185 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Anti-retrovirals: Do not take anti-retroviral medications for at least one month prior to initiating medications for stem cell mobilization and for the expected duration of time needed for the elimination of the medications. ( 7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation before LENMELDY Infusion
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis and Thromboembolic Events
5.2 Encephalitis
5.3 Serious Infection
5.4 Veno-occlusive Disease
5.5 Delayed Platelet Engraftment
5.6 Neutrophil Engraftment Failure
5.7 Insertional Oncogenesis
5.8 Hypersensitivity Reactions
5.9 Anti-retroviral Use
5.10 Interference with Serology Testing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Vaccines
7.2 Anti-retrovirals
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.6 Patients Seropositive for Human Immunodeficiency Virus (HIV) or other infectious diseases
8.7 Renal Impairment
8.8 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For one-time single-dose intravenous use only.
2.1 Dose
LENMELDY is provided as a single dose for infusion containing a suspension of CD34 +cells in one to eight infusion bags. Table 1 provides the minimum and maximum recommended dose of LENMELDY based on MLD disease subtype:
Table 1: Minimum and Maximum Recommended Dose of LENMELDY MLD Subtype Minimum Recommended Dose
(CD34 +cells/kg)Maximum Recommended Dose
(CD34 +cells/kg)Pre-symptomatic late infantile 4.2 x 10 6 30 x 10 6 Pre-symptomatic early juvenile 9 x 10 6 30 x 10 6 Early symptomatic early juvenile 6.6 x 10 6 30 x 10 6 The dose administered is calculated based on the child’s weight at time of LENMELDY infusion using the information provided on the Lot Information Sheet. See the Lot Information Sheet provided with the product shipment for additional information pertaining to dose.
2.2 Preparation before LENMELDY Infusion
Mobilization, apheresis, and myeloablative conditioning are required prior to LENMELDY infusion. Before initiating these procedures, confirm that hematopoietic stem cell (HSC) gene therapy is appropriate for the child.
Screen children for hepatitis B virus (HBV), hepatitis C virus (HCV), human T-lymphotrophic virus 1 & 2 (HTLV-1/HTLV-2), human immunodeficiency virus 1 & 2 (HIV-1/HIV-2), cytomegalovirus (CMV), and mycoplasma infection in accordance with clinical guidelines before collection of cells for manufacturing.
Mobilization and Apheresis
Children are required to undergo HSC mobilization followed by apheresis to obtain CD34 +cells for LENMELDY manufacturing. In clinical trials of LENMELDY, granulocyte-colony stimulating factor (G-CSF) with or without plerixafor was used for mobilization.
For the manufacture of LENMELDY, a collection of a minimum of 8.0 × 10 6CD34 +cells/kg of autologous cells is required based on a weight at time of apheresis collection. Collection of the minimum number of CD34 +cells required for manufacture may be achieved using one or more cycles of mobilization.
A collection of unmanipulated back-up CD34 +cells of at least 2.0 × 10 6CD34 +cells/kg is required. These cells must be collected from the child and be cryopreserved prior to myeloablative conditioning. The back-up collection may be needed for rescue treatment if there is: 1) compromise of LENMELDY after initiation of conditioning but before infusion, 2) primary engraftment failure, or 3) loss of engraftment after infusion with LENMELDY. The back-up cells may be collected either through mobilized peripheral blood (mPB) apheresis or bone marrow collection.
Myeloablative Conditioning
Myeloablative conditioning must be administered before infusion of LENMELDY. In clinical trials of LENMELDY, busulfan was used for myeloablative conditioning. There is no data available supporting the use of alternative conditioning agents with LENMELDY.
Do not begin myeloablative conditioning until LENMELDY has been received and stored at the treatment center and the availability of the back-up collection of CD34 +cells has also been confirmed. After completion of the myeloablative conditioning, allow a minimum of 24 hours of washout before LENMELDY infusion.
Receipt and Storage of LENMELDY
- LENMELDY is shipped to the treatment center in the vapor phase of liquid nitrogen at less than -130°C (-202°F) along with the corresponding Lot Information Sheet. Two shippers would be used in the event that 5-8 bags are manufactured.
-
Confirm patient identifiers on the product labels and the Lot Information Sheet.
- If there are any concerns about the product or packaging upon receipt, contact Orchard Therapeutics at 1-888-878-0185.
- Keep the infusion bag(s) in the metal cassette(s) and transfer LENMELDY from the transport vapor phase of liquid nitrogen shipper to the treatment center’s own vapor phase of liquid nitrogen storage at less than -130°C (-202°F). Store in the vapor phase of liquid nitrogen at less than -130°C (-202°F) until ready for thaw and administration.
Preparation of LENMELDY for Infusion
Coordinate the timing of LENMELDY thaw and infusion. Confirm the infusion time in advance and adjust the start time of LENMELDY thaw such that it will be available for infusion when the child and healthcare providers are ready to initiate LENMELDY administration as soon as possible after thaw. Each bag of LENMELDY should be infused via a central venous catheter and must be infused within 2 hours, post-thawing.
LENMELDY contains human blood cells that are genetically modified with replication-incompetent, self-inactivating lentiviral vector (LVV). Follow universal precautions and local biosafety guidelines for handling and disposal of LENMELDY to avoid potential transmission of infectious diseases.
Ensure the correct number of infusion bags are present. Use the corresponding Lot Information Sheet to confirm the number of LENMELDY bags shipped to the treatment center. A maximum of eight bags may be provided per child, which would take a maximum of approximately 16 hours to complete the infusion process. If more than one infusion bag is provided, thaw and administer each infusion bag completely before proceeding to thaw the next infusion bag. The following steps must be repeated for each LENMELDY infusion bag immediately prior to thaw.
- Remove the metal cassette from the vapor phase of liquid nitrogen storage.
- Confirm that "LENMELDY" is printed on the infusion bag label.
-
Confirm that the child's identity matches the unique patient identifiers located on the LENMELDY infusion bag. Do not infuse LENMELDY if the information on the patient-specific label on the infusion bag does not match the intended patient, and contact Orchard Therapeutics at 1-888-878-0185.
- Use the accompanying Lot Information Sheet to confirm that the infusion bag is within the expiration date.
- Inspect the infusion bag for any breaches of integrity before thawing and infusion. If an infusion bag is compromised, follow the local guidelines and contact Orchard Therapeutics immediately at 1-888-878-0185.
- Thaw LENMELDY in the overwrap bag at 37°C (98.6°F) in a controlled thawing device. Once thawing is complete, the bag should be removed immediately from the thawing device. The overwrap bag should be carefully opened to remove the infusion bag which should be kept at room temperature until infusion.
- After thawing, mix the contents gently by massaging the infusion bag to homogenize the cell suspension and disperse any remaining cell aggregates. If visible cell aggregates remain, continue to gently mix the contents of the bag. Most cell aggregates should disperse with gentle manual mixing. Do not shake the bag. Do not wash, spin down and/or resuspend LENMELDY in new media prior to infusion.
- Do not sample, alter, irradiate, or refreeze LENMELDY.
2.3 Administration
LENMELDY is for autologous use only. The child’s identity must match the patient identifiers on the LENMELDY cassette(s) and infusion bag(s). Do not infuse LENMELDY if the information on the patient-specific label does not match the intended patient. LENMELDY must not be irradiated or infused using a leukodepleting filter.
-
Before infusion, confirm that the child’s identity matches the unique patient identifiers on the LENMELDY infusion bag.Use the Lot Information Sheet to confirm the total number of infusion bags to be administered.
- Prime the tubing of the infusion set with 0.9% sodium chloride solution prior to infusion.
- Expose the sterile port on the infusion bag by tearing off the protective wrap covering the port.
- Access the infusion bag and infuse LENMELDY as soon as possible after thawing and
complete the infusion within 2 hours after thawing.
- Administer each infusion bag of LENMELDY as an intravenous infusion via a central venous catheter within 30 minutes via gravity or infusion pump. An infusion flow rate should be calculated based on the volume in each infusion bag.
- After the entire content of the infusion bag is infused, flush all LENMELDY remaining in the infusion bag and any associated tubing with 0.9% sodium chloride solution using a rinse volume that is equal to or greater than the priming volume of any intravenous infusion set used to ensure that as many cells as possible are infused into the child.
- If more than one infusion bag is provided, administer the content of each infusion bag completely before proceeding to thaw (following Section
2.2steps 1-7) and infuse (following Section
2.3steps 1-6) the content of the next infusion bag. If more than one infusion bag is necessary, do not administer more than one bag of product per hour.
After LENMELDY Administration
Standard procedures for patient management after HSC transplantation should be followed after LENMELDY infusion.
- Irradiate any blood products required within the first 3 months after LENMELDY infusion.
- Children treated with LENMELDY should not donate blood, organs, tissues, or cells at any time in the future.
- LENMELDY is shipped to the treatment center in the vapor phase of liquid nitrogen at less than -130°C (-202°F) along with the corresponding Lot Information Sheet. Two shippers would be used in the event that 5-8 bags are manufactured.
-
3 DOSAGE FORMS AND STRENGTHS
LENMELDY is a single-dose cell suspension for intravenous infusion.
LENMELDY is composed of one to eight infusion bags which contain 2 to 11.8 × 10 6cells/mL (1.8 to 11.8 x 10 6CD34 +cells/mL) suspended in cryopreservation solution [ see How Supplied/Storage and Handling (16)]. Each infusion bag contains 10 to 20 mL of LENMELDY.
See the Lot Information Sheet for actual dose.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis and Thromboembolic Events
Treatment with LENMELDY may increase the risk of thrombosis and thromboembolic events. A child with PSEJ MLD died after experiencing a left hemisphere cerebral infarction secondary to a thrombotic event in a large blood vessel approximately one year after treatment with LENMELDY. Prior to the cerebral infarction, the child’s D-dimer was elevated (82 nmol/L, normal range: 1.5 - 4.2). Additional clinical findings included a minor elevation of liver enzymes. The etiology of the cerebral infarction was unclear but attribution to LENMELDY cannot be ruled out. No other events related to cerebral infarction have been reported during the clinical development of LENMELDY.
Some children received anti-thrombotic prophylaxis [ see Clinical Trials Experience (6.1)]. Evaluate the risk factors for thrombosis prior to and after LENMELDY infusion according to best clinical practice.
5.2 Encephalitis
Treatment with LENMELDY may increase the risk of encephalitis. A child with ESEJ MLD developed a serious event of encephalitis after treatment with LENMELDY. At the time of treatment, GMFC-MLD was Level 1 (able to walk independently with impaired gait). One month after treatment, the child experienced subacute neurological deterioration, with asthenia, hypotonia, cognitive and behavioral problems, vomiting, and swallowing disturbance. The child was afebrile, blood and CSF cultures were negative for bacterial infection, a large viral panel was negative and all routine laboratory tests were normal. The child was treated with plasmapheresis, immunoglobulin and rituximab leading to clinical improvement. Five months after onset of symptoms the child had reached GMFC-MLD Level 4 (walking not possible; able to sit without support and locomotion possible, or unable to sit without support but locomotion is possible). The child was subsequently treated with eculizumab and tocilizumab.
The etiology of this event is unclear but attribution to LENMELDY cannot be ruled out. Treatment with LENMELDY may trigger a relapsing-remitting pattern of disease progression. No other events related to encephalitis have been reported during the clinical development of LENMELDY.
Monitor children for signs or symptoms of encephalitis after LENMELDY treatment.
5.3 Serious Infection
In the period between start of conditioning and within one year after LENMELDY treatment, severe Grade 3 infections occurred in 39% of all children (21% bacterial, 5% viral, 5% bacterial and viral or bacterial and fungal, and 8% unspecified). The most common Grade 3 infections were device related infections (18%) (including two events of sepsis), respiratory tract infections (including 1 Grade 3 event of pneumonia) (8%), and gastroenteritis/enteritis (8%).
Grade 3 febrile neutropenia developed within 1 month after LENMELDY infusion in 82% of children. In the event of febrile neutropenia, monitor for signs and symptoms of infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
Monitor children for signs and symptoms of infection after myeloablative conditioning and LENMELDY infusion and treat appropriately. Administer prophylactic antimicrobials according to best clinical practice.
5.4 Veno-occlusive Disease
Three children (8%) treated in clinical trials of LENMELDY developed veno-occlusive disease (VOD), with one Grade 4 SAE and two Grade 3 AEs. None of these three events met Hy’s Law criteria.
Monitor children for signs and symptoms of VOD including liver function tests in all children during the first month after LENMELDY infusion. Consider prophylaxis for VOD with anti-thrombotic agents based on risk factors for VOD and best clinical practice.
5.5 Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with LENMELDY treatment [ see Adverse Reactions (6.1)]. Bleeding risk is increased prior to platelet engraftment and may continue after engraftment in children with prolonged thrombocytopenia. In clinical trials of LENMELDY, 4 (10%) children had delayed platelet engraftment after day 60 (range day 67-109), with 3 children requiring platelet transfusions until engraftment occurred. All children treated with LENMELDY received transfusion support with platelets according to best clinical practice.
Inform children of the risk of bleeding until platelet recovery has been achieved. Monitor children for thrombocytopenia and bleeding.
5.6 Neutrophil Engraftment Failure
There is a potential risk of neutrophil engraftment failure after treatment with LENMELDY. In clinical trials of LENMELDY, no cases of neutrophil engraftment failure have been reported. Neutrophil engraftment failure is defined as failure to achieve three consecutive absolute neutrophil counts (ANC) ≥ 500 cells/microliter obtained on different days by Day 60 after infusion of LENMELDY.
Monitor neutrophil counts until engraftment has been achieved. If neutrophil engraftment failure occurs in a child treated with LENMELDY, provide rescue treatment with the unmanipulated back-up collection of CD34 +cells [ see Preparation before LENMELDY Infusion (2.2)].
5.7 Insertional Oncogenesis
There is a potential risk of LVV-mediated insertional oncogenesis after treatment with LENMELDY. In clinical trials of LENMELDY, no cases of insertional oncogenesis have been reported. Children treated with LENMELDY may develop hematologic malignancies and should be monitored lifelong. Monitor for hematologic malignancies with a complete blood count (with differential) annually and integration site analysis as warranted for at least 15 years after treatment with LENMELDY.
In the event that a malignancy occurs, contact Orchard Therapeutics at 1-888-878-0185 for reporting and to obtain instructions on collection of samples for testing.
5.8 Hypersensitivity Reactions
There is a potential risk of allergic reactions in children treated with LENMELDY. The dimethyl sulfoxide (DMSO) in LENMELDY may cause hypersensitivity reactions, including anaphylaxis which is potentially life-threatening and requires immediate intervention. Hypersensitivity including anaphylaxis can occur in children with and without prior exposure to DSMO. In clinical trials of LENMELDY, no cases of hypersensitivity reactions have been reported.
5.9 Anti-retroviral Use
Children should not take prophylactic HIV anti-retroviral medications for at least one month prior to mobilization, or for the expected duration of time needed for the elimination of the medications. Anti-retroviral medications may interfere with the manufacturing of LENMELDY. [ see Drug Interactions (7.2)].
If a child requires anti-retrovirals for HIV prophylaxis, initiation of LENMELDY treatment should be delayed until confirmation of a negative test for HIV.
5.10 Interference with Serology Testing
Children who have received LENMELDY are likely to test positive by polymerase chain reaction (PCR) assays for HIV due to LVV provirus insertion resulting in a false-positive test for HIV. Therefore, children who have received LENMELDY should not be screened for HIV infection using a PCR-based assay.
-
6 ADVERSE REACTIONS
The most common non-laboratory adverse reactions (occurring in ≥10% of all children within Year 1 of treatment) were: febrile neutropenia (85%), stomatitis (77%), respiratory tract infections (54%), rash (33%), device related infections (31%), other viral infections (28%), pyrexia (21%), gastroenteritis (21%), and hepatomegaly (18%).
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thrombosis and Thrombotic Events [ see Warnings and Precautions (5.1)]
- Encephalitis [ see Warnings and Precautions (5.2)]
- Serious Infection [ see Warnings and Precautions (5.3)]
- Veno-occlusive Disease [ see Warnings and Precautions (5.4)]
- Delayed Platelet Engraftment [ see Warnings and Precautions (5.5)]
- Neutrophil Engraftment Failure [ see Warnings and Precautions (5.6)]
- Insertional Oncogenesis [ see Warnings and Precautions (5.7)]
- Hypersensitivity Reactions [ see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data reflect experience from 39 children with MLD treated in clinical trials of LENMELDY: PSLI (n=20), PSEJ (n=7), ESEJ (n=10), and 2 children with advanced disease at the time of treatment [ see Clinical Studies (14)]. The median (min, max) years of follow-up for the safety population was 6.8 (0.6, 12.2).
Table 2 presents the non-laboratory treatment emergent adverse reactions occurring in ≥10% of all children with onset reported on or after the date of conditioning up to 1 year follow-up post-treatment.
Table 2: Summary of Non-Laboratory Treatment Emergent Adverse Reactions Reported in at Least 10% of Patients Within Year 1 Following Treatment with LENMELDY (N = 39) * Adverse reaction
System Organ Class/Preferred TermAny Grade
n (%) patientsGrade 3 or higher
n (%) patents- *
- Includes adverse events potentially related to busulfan myeloablative conditioning.
- †
- Includes events with PTs of Bronchitis, Nasopharyngitis, Pharyngitis, Pneumonia, Respiratory tract infection, Rhinitis, Tonsillitis, Upper respiratory fungal infection and Upper respiratory tract infection.
- ‡
- Includes events of Bacterial sepsis, Catheter site cellulitis, Catheter site infection, Device related infection, Sepsis, and Vascular device infection.
- §
- Includes events with PTs of Enteritis, Gastroenteritis, Gastroenteritis Aeromonas and Gastroenteritis rotavirus.
- ¶
- Includes events with PTs of Adenovirus infection, Cytomegalovirus infection, Cytomegalovirus test positive, Cytomegalovirus viremia, Enterovirus infection, Hand-foot-and-mouth disease, Herpes zoster, SARSCov-2-test positive, and Viral infection (excluding PT of Gastroenteritis rotavirus).
- #
- Includes events with PTs of Dermatitis, Dermatitis bullous, Rash, Rash erythematous, Drug eruption and Rash maculopapular.
Blood and lymphatic system disorders --
--
Febrile neutropenia 33 (85) 32 (82) Gastrointestinal disorders --
--
Stomatitis 30 (77) 29 (74) General disorders and administration site conditions --
--
Pyrexia 8 (21) 1 (3) Hepatobiliary disorders --
--
Hepatomegaly 7 (18) 0 Infections and infestations --
--
Respiratory tract infections † 21 (54) 3 (8) Device related infection ‡ 12 (31) 7 (18) Gastroenteritis § 8 (21) 3 (8) Other viral infections ¶ 11 (28) 2 (5) Skin and subcutaneous tissue disorders --
--
Rash # 13 (33) 3 (8) In clinical trials of LENMELDY, 11 children (28%) developed an adverse event of neutropenia following conditioning, 8 of which (21%) reported a Grade 3 event. Despite best clinical practices including transfusion to prevent severe anemia, three children (8%) developed an adverse event of anemia, 1 of which reported a Grade 3 event. Nine children (23%) developed elevated liver enzymes, including three children (8%) who developed a Grade 3 event. Elevated levels of D-dimer (greater than 4.2 nmol/L) have been reported in 26/39 (67%) children during clinical trials of LENMELDY. Abnormal values up to 109.5 nmol/L were identified at timepoints from Day 21 to Year 7 after LENMELDY infusion, with no pattern identified.
Other clinically important events that occurred in < 10% of the population included the following:
One child reported Grade 3 adverse reactions of elevated alanine- and aspartate transaminases which met the definition of Hy’s Law at day 14 after LENMELDY infusion and resolved without treatment by 9 months after initial onset. Two children reported Grade 3 elevations in liver enzymes, and 1 child reported Grade 1 elevations in liver enzymes. All events resolved without treatment and are considered related to conditioning.
-
7 DRUG INTERACTIONS
No formal drug interaction studies have been performed. LENMELDY is not expected to interact with the hepatic cytochrome P-450 family of enzymes or drug transporters.
7.1 Vaccines
The safety and effectiveness of vaccination during or following LENMELDY treatment have not been studied. Vaccination is not recommended during the 6 weeks preceding the start of myeloablative conditioning, and until hematological recovery following treatment with LENMELDY. Where feasible, administer childhood vaccinations prior to myeloablative conditioning for LENMELDY.
7.2 Anti-retrovirals
Children should not take anti-retroviral medications for at least one month prior to mobilization or the expected duration for elimination of the medications [ see Warnings and Precautions (5.5)]. Anti-retroviral medications may interfere with the manufacturing of LENMELDY.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no clinical data from the use of LENMELDY in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with LENMELDY to assess whether it can cause fetal harm when administered to a pregnant woman. LENMELDY must not be administered during pregnancy because of the risk associated with myeloablative conditioning. Pregnancy after LENMELDY infusion should be discussed with the treating physician.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of LENMELDY in human or animal milk, the effects on the breastfed child, or the effects on milk production. Because of the potential risks associated with myeloablative conditioning, breast-feeding should be discontinued during conditioning. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LENMELDY and any potential adverse effects on the breastfed child from LENMELDY or from the underlying maternal condition. Breast-feeding after LENMELDY infusion should be discussed with the treating physician. Children receiving breast milk may continue to do so throughout their treatment under the advice of their treating physician.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
As a precautionary measure, a negative serum pregnancy test must be confirmed prior to the start of mobilization and re-confirmed prior to conditioning procedures and before administration of LENMELDY in females of childbearing potential.
Contraception
Males capable of fathering a child and females of childbearing age should use an effective method of contraception from start of mobilization through at least 6 months after administration of LENMELDY.
Infertility
There are no data on the effects of LENMELDY on fertility.
Data are available on the risk of infertility with myeloablative conditioning. In clinical trials of LENMELDY, seven children (50% of females) developed ovarian failure. Advise children of the option to cryopreserve semen or ova before treatment, if appropriate.
8.4 Pediatric Use
The safety and efficacy of LENMELDY has been established in children with PSLI, PSEJ and ESEJ MLD. The clinical trials of LENMELDY treated 20 PSLI, 7 PSEJ, and 10 ESEJ MLD children who received LENMELDY between ages 8 – 19 months (median age of 12 months), 11 months – 5.56 years (median age of 2.57 years), and 2.54 – 11.64 years (median age of 5.84 years), respectively. The safety and efficacy of LENMELDY have not yet been established in children with the late juvenile form of the disease [ see Clinical Studies (14)].
8.6 Patients Seropositive for Human Immunodeficiency Virus (HIV) or other infectious diseases
LENMELDY has not been studied in children with HIV-1, HIV-2, HTLV-1, HTLV-2, HBV, HVC, or mycoplasma infection. Negative serology tests for HIV-1/2, HTLV-1/2, HBV, HCV, and mycoplasma are necessary to ensure acceptance of apheresis material for LENMELDY manufacturing.
-
11 DESCRIPTION
LENMELDY (atidarsagene autotemcel) is a gene therapy consisting of autologous CD34 +cells, containing hematopoietic stem cells (HSCs), transduced with a lentiviral vector (LVV) encoding the human arylsulfatase A (ARSA) gene, suspended in cryopreservation solution. LENMELDY is intended for one-time administration to add functional copies of the ARSA gene into the child’s own HSCs.
LENMELDY is prepared from the child’s own HSCs, which are collected via apheresis procedure(s). The autologous cells are enriched for CD34 +cells, then transduced ex vivowith a recombinant replication-incompetent self-inactivating (SIN) human immunodeficiency virus-1 (HIV-1) - based LVV that has been modified to carry the ARSA cDNA sequence under the human phosphoglycerate kinase (PGK) promoter. The transduced CD34 +cells are washed, formulated into a suspension, and then cryopreserved.
LENMELDY is manufactured for each individual child into infusion bags, which are cryopreserved before being thawed prior to administration [ see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)]. The thawed product is a colorless to slightly yellow or pink cell suspension and may contain visible cell aggregates.
The formulation contains 5% (v/v) dimethyl sulfoxide (DMSO).
Each 1 mL of LENMELDY suspension for IV infusion contains 3.5 mg of sodium.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
LENMELDY inserts one or more functional copies of the human ARSA complementary deoxyribonucleic acid (cDNA) into the patients’ HSCs, through transduction of autologous CD34 +cells with ARSA LVV. After LENMELDY infusion, transduced CD34 +HSCs engraft in bone marrow, repopulate the hematopoietic compartment and their progeny produce ARSA enzyme. Functional ARSA enzyme can breakdown or prevent the harmful accumulation of sulfatides.
12.2 Pharmacodynamics
Deficiency of ARSA is known to be the cause of MLD; therefore, ARSA activity in peripheral blood mononuclear cells of the hematopoietic lineage (i.e. ARSA in PBMC) was evaluated to provide evidence of pharmacodynamic activity of LENMELDY.
Median ARSA activity in PBMCs was at supranormal levels by 3 months post-treatment in the PSLI and PSEJ populations (normal range for ARSA activity is 31-198 nmol/mg/h) and supranormal median levels were sustained throughout the duration of follow-up (Table 3). In ESEJ median ARSA activity was within normal range from Month 3 to Year 2 and supranormal activity was achieved at Year 3 and 5 (Table 3).
Table 3: Summary of ARSA Activity (nmol/mg/h) in Total Peripheral Blood Mononuclear Cells by Visit in the Patients Treated with LENMELDY Visit Variable PSLI (N=20) PSEJ (N=7) ESEJ (N=10) - *
- One value was below the lower limit of quantification (LLQ) or not detected or not quantifiable, and has been imputed as the LLQ (26 nmol/mg/h). Normal range for ARSA activity is 31-198 nmol/mg/h. ARSA=Arylsulfatase A; Max=Maximum; Min=Minimum. Number of children at timepoint represents the number of children with non-missing data (including those with imputed values).
Month 3 Patient Samples, n 18 6 10 -- Median
(Min, Max)682
(61, 3398)1140
(314, 1300)210
(50, 426)Month 6 Patient Samples, n 16 6 7 * -- Median
(Min, Max)1095
(37, 2716)983
(150, 1804)107
(26, 444)Year 1 Patient Samples, n 20 7 8 -- Median
(Min, Max)1239
(46, 6467)883
(272, 1976)130
(55, 688)Year 2 Patient Samples, n 18 * 6 7 -- Median
(Min, Max)935
(26, 5935)1063
(328, 2205)82
(70, 219)Year 3 Patient Samples, n 18 * 4 6 -- Median
(Min, Max)1558
(26, 7091)1156
(537, 2173)234
(30, 1271)Year 5 Patient Samples, n 9 0 3 -- Median
(Min, Max)756
(28, 3474)- 363
(282, 793)12.3 Pharmacokinetics
LENMELDY is an autologous gene therapy which includes hematopoietic stem cells (HSCs) that have been genetically modified ex vivo. The nature of LENMELDY is such that conventional pharmacokinetic studies on absorption, distribution, metabolism, and excretion are not applicable.
12.6 Immunogenicity
During the clinical development program, anti-ARSA antibodies (AAA) were reported in 6/39 children (5 PSLI and 1 PSEJ) with titers between 1:80 and 1:6400. Five events resolved between Days 45 and 368, of which two resolved spontaneously. One event was reported as ongoing. One child reported declining ARSA levels in the setting of AAA but the impact on clinical efficacy is unknown as the child is still in the pre-symptomatic phase of the disease. Four children received treatment with rituximab. There is insufficient information to characterize the impact of AAA on ARSA activity in PBMC, clinical efficacy, or safety.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The safety and efficacy of LENMELDY was assessed in 39 children across two single-arm, open-label clinical trials and a European Union (EU) expanded access program (EAP). Two children with advanced disease were excluded from the efficacy analysis. The clinical trials enrolled 13 children with PSLI, 6 children with PSEJ and 9 children with ESEJ MLD. The EU EAP enrolled 7 children with PSLI, 1 child with PSEJ and 1 child with ESEJ MLD. All children had documented biochemical and molecular diagnosis of MLD based on ARSA activity below the normal range and identification of two disease-causing ARSAalleles. In the case of a novel ARSAvariant(s), a 24-hour urine collection was required to show elevated sulfatide levels.
The major efficacy outcomes in clinical trials of LENMELDY were motor and neurocognitive function, as assessed by GMFC-MLD levels and standard scores on age-appropriate neurocognitive tests, respectively. The efficacy of LENMELDY was compared to an external untreated natural history (NHx) cohort of children with LI (n=28) and EJ (n=21) MLD. Data from the NHx cohort were collected both retrospectively and prospectively. Cognitive outcomes in the children with PSEJ and ESEJ MLD were compared to outcomes for untreated children reported in the medical literature.
In clinical trials of LENMELDY, children were classified as having PSLI, PSEJ, or ESEJ MLD based on the following criteria:
- PSLI MLD: Children with expected disease onset ≤ 30 months of age and an ARSAgenotype consistent with LI MLD. Pre-symptomatic status* defined as the absence of neurological signs and symptoms of MLD.
- PSEJ MLD: Children with expected disease onset > 30 months and <7 years of age and an ARSAgenotype consistent with EJ MLD. Pre-symptomatic status* defined as the absence of neurological signs and symptoms of MLD or physical exam findings limited to abnormal reflexes and/or clonus.
- ESEJ MLD: Children with disease onset > 30 months and <7 years of age and an ARSAgenotype consistent with EJ MLD. Early symptomatic status defined as walking independently (GMFC-MLD Level 0 with ataxia or GMFC-MLD Level 1) and IQ ≥ 85.
*Pre-symptomatic children were permitted to have abnormal reflexes or abnormalities on brain magnetic resonance imaging and/or nerve conduction tests not associated with functional impairment (e.g., no tremor, no peripheral ataxia).
Demographics and baseline characteristics for children who were included in the LENMELDY Integrated Summary of Efficacy analyses (n=37) are shown in Table 4.
Table 4: Demographics and Baseline Characteristics for Children with PSLI, PSEJ and ESEJ MLD Treated with LENMELDY (N=37) Parameter PSLI (N=20) PSEJ (N=7) ESEJ (N=10) Age at Treatment (months) - Median
(Min, Max)12
(8, 19)31
(11, 67)70
(31, 140)Male – n (%) 13 (65) 6 (86) 6 (60) Race – n (%) -- -- -- White/Caucasian 18 (90) 6 (86) 10 (100) Asian 2 (10) 0 0 Black or African American 0 1 (14) 0 Ethnicity - Hispanic or Latino – n (%) 1 (5) 0 0 HSC Collection:
HSCs used for the manufacture of LENMELDY were obtained by bone marrow (BM) collection (n=29), from apheresis collection of peripheral blood following the administration of HSC mobilizing agents (mPB) (n=8), or HSCs were obtained from both sources (n=2). Mobilization was achieved using G-CSF administered twice daily. From Day 3, plerixafor could be administered once daily. In the clinical trials of LENMELDY, plerixafor was administered to the 8 children where HSCs were obtained from apheresis collection only.
Pre-treatment Conditioning and Supportive Care:
All children received busulfan conditioning with a total dose range of 9 to 32 mg/kg and a target cumulative area under the curve (AUC) of 58,800 to 93,500 μg*h/L. Defibrotide was used in 11/39 children as prophylaxis for VOD. No additional anti-thrombotic agents were used as prophylaxis for VOD.
LENMELDY administration:
The ranges of doses administered associated with clinical efficacy are presented in Table 5.
Table 5: Doses of LENMELDY Associated with Clinical Efficacy in Children with PSLI, PSEJ, and ESEJ MLD MLDSubtype Median Dose
(x 10 6CD34+
cells/kg)Min, Max Dose
(x 10 6CD34+
cells/kg)Minimum
Weight
(kg)Minimum Age
at Treatment
(months)- *
- LENMELDY has been administered to children with PSEJ MLD from 12 months of age, but efficacy has not been determined due to limited follow up post-treatment.
- †
- Higher doses (up to 30 x 10 6 CD34+ cells/kg) have been administered to children with ESEJ MLD but efficacy has not been determined due to limited follow up post-treatment.
Pre-symptomatic late infantile 14.2 4.2, 30 7.2 8 Pre-symptomatic early juvenile 9.7 9, 30 14.5 43 * Early-symptomatic early juvenile 9.5 6.6, 10.9 † - - One child with ESEJ MLD was treated with a dose of 6 x 10 6CD34 +cells/kg and did not show evidence of efficacy.
Comparison of LENMELDY Treatment with the Natural History of MLD in PSLI, PSEJ, and ESEJ
PSLI
Severe Motor Impairment-Free Survival (sMFS)
The primary endpoint was severe motor impairment-free survival, defined as the interval from birth to the first occurrence of loss of locomotion and loss of sitting without support (GMFC-MLD Level ≥ 5) or death. For analyses of the primary endpoint, an additional 2 untreated siblings not enrolled in the NHx study were included in the comparator group. Treatment with LENMELDY significantly extended severe motor impairment-free survival in children with PSLI MLD compared with untreated LI natural history children (Figure 1).
Figure 1: Kaplan-Meier Curve of Severe Motor Impairment-Free Survival for Children with PSLI MLD Treated with LENMELDY and LI Natural History Populations
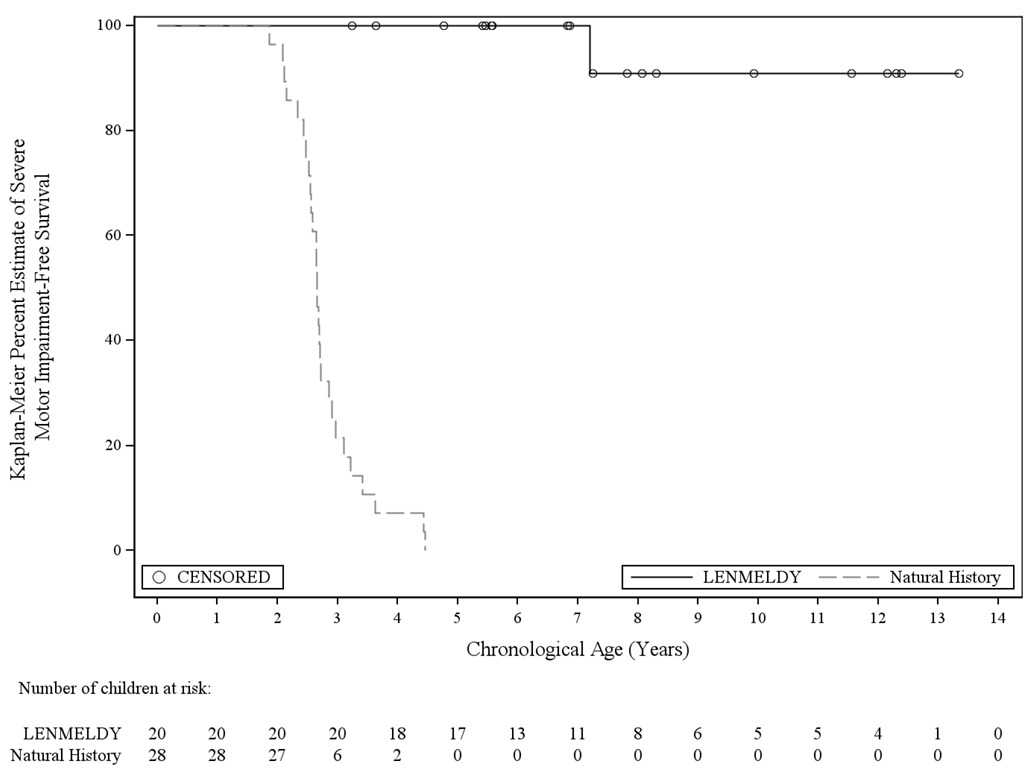
- Seventeen children with PSLI MLD treated with LENMELDY have been followed until at least the age of 5 years. At the age of 5 years, 100% of LENMELDY treated PSLI children remained event-free compared with 0% of untreated LI children.
- Twelve out of 17 children who were at least 5 years of age at last follow-up (ages 5.4-13.3 years of age) retained independent ambulation (GMFC-MLD Level ≤ 1).
- Two children at the time of last assessment (ages 8.1 and 11.6 years) were able to ambulate with support (GMFC-MLD Level 2). Loss of ambulation without support occurred at 3.6 and 7.8 years of age, respectively.
- One child had progressed to GMFC-MLD Level 5 (loss of locomotion and loss of sitting without support; severe motor impairment) by age 7.2 years and lost all motor function (GMFC-MLD Level 6) at age 9.9 years.
- Two children never achieved independent ambulation.
Overall Survival
Treatment with LENMELDY significantly extends overall survival compared to untreated natural history. Fourteen treated children and 24 natural history children had sufficient follow-up to determine survival at 6 years from birth. At this timepoint, all patients treated with LENMELDY were alive, and 10 natural history children had died (42%).
Cognitive Function
Cognitive function was captured by neuropsychological tests (Bayley Scale of Infant Development [BSID], Wechsler Preschool and Primary Scale of Intelligence [WPPSI], Wechsler Intelligence Scale for Children [WISC] or Wechsler Adult Intelligence Scale [WAIS]), according to the child’s age and/or ability. Where required, due to the treated child’s severe decline and/or the limitations of the natural history evidence, developmental quotient scores were derived from age equivalent scores. When assessed within the appropriate age ranges, a standard score can be derived, allowing comparison of a child’s cognitive ability with the normative population. Cognitive function was defined using the following: normal cognitive function, standard score ≥ 85; mild cognitive impairment, standard score ≥ 70 and < 85; moderate cognitive impairment, score >55 and < 70; severe cognitive impairment, score ≤ 55.
Performance and language standard scores for children with PSLI MLD treated with LENMELDY compared to LI NHx children are presented in Figure 2 and Figure 3, respectively. Where a child has been assessed utilizing an age-appropriate test, and a standard score has been obtained, these are presented as closed circles for children with PSLI MLD treated with LENMELDY and as closed triangles for LI NHx children. Where an age appropriate standard score could not be obtained due to cognitive impairment, the standard score has been imputed as zero (open circles for children with PSLI MLD treated with LENMELDY and open triangles for LI NHx children).
Nineteen of 20 children with PSLI MLD had performance standard scores above the threshold of severe cognitive impairment (performance standard score > 55) through to the last follow-up. At last assessment, two of these children were below the threshold for moderate cognitive impairment (< 70), with all others maintaining performance standard scores ≥ 70 and most maintaining normal scores (≥ 85). This contrasts markedly with results in LI NHx children with completed neuropsychological assessments who demonstrate severe cognitive impairment early in their disease course (Figure 2).
Figure 2: Plot of Performance Standard Score vs. Age (PSLI) LENMELDY and LI Natural History Populations
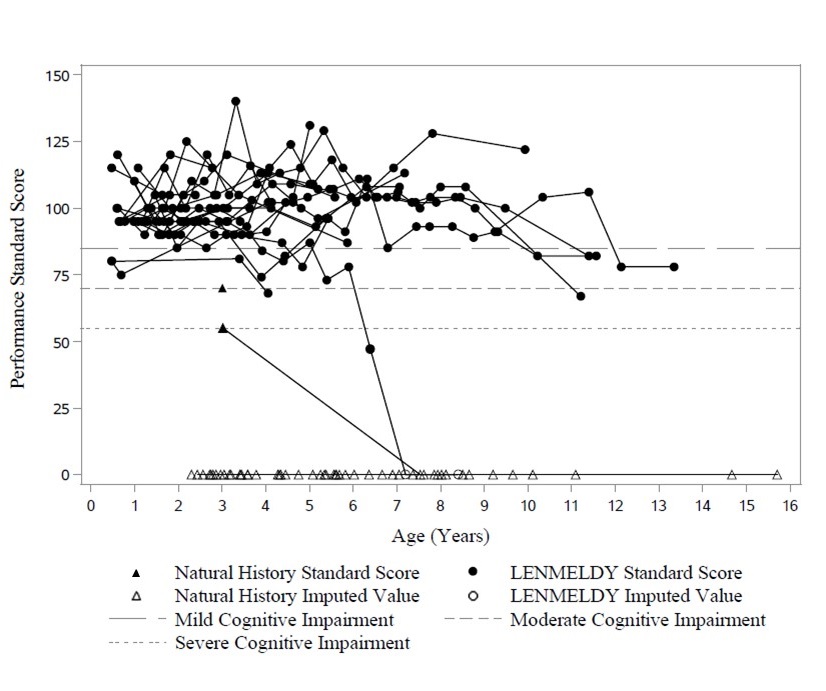
Note: Performance standard scores have been imputed as zero in cases where they could not be derived due to severe cognitive impairment.
Nineteen of 20 children with PSLI MLD treated with LENMELDY had language standard scores above the threshold of severe impairment (language standard scores > 55) at last follow-up. At last assessment, two of these children were just below the threshold for moderate impairment (< 70), with all others maintaining language standard scores ≥ 70 and most maintaining normal scores (≥ 85). This contrasts markedly with results in LI NHx children with completed neuropsychological assessments who demonstrate severe impairment early in their disease course (Figure 3).
Figure 3: Plot of Language Standard Score vs. Age (PSLI) LENMELDY and LI Natural History Populations
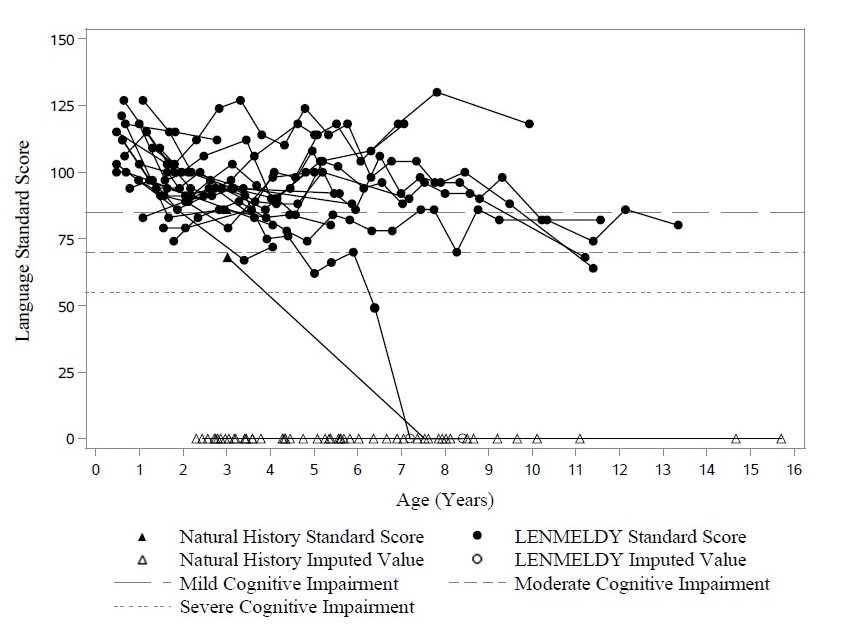
Note: Language standard scores have been imputed as zero in cases where they could not be derived due to severe cognitive impairment.
PSEJ
Seven children with PSEJ MLD were treated with LENMELDY. One child died at age 2.1 years from a cerebral infarction. There were insufficient data in three children who were too young at last follow-up to evaluate efficacy of LENMELDY as symptom onset may not begin until 7 years of age in EJ MLD. Two children had evaluable motor and cognitive outcomes. One child had evaluable motor outcomes, but while showing stable normal cognitive function, was neither old enough nor had sibling data for cognitive events to be evaluable.
Motor Function
Three of seven children had evaluable motor outcomes:
- One child treated at 4.1 years of age retained normal gait (GMFC-MLD Level 0) at age 11.9 years. Their matched sibling comparator lost all motor function (GMFC-MLD Level 6) by age 6 years.
- One child treated at 3.6 years of age retained normal gait (GMFC-MLD Level 0) at age 7.3 years. Their matched sibling comparator developed impaired gait (GMFC-MLD Level 1) at age 5 years.
- One child treated at 5.6 years of age retained normal gait (GMFC-MLD Level 0) until age 12.8 years and still had independent ambulation (GMFC-MLD Level 1) at 13.6 years of age.
Cognitive Function
Two of seven children with PSEJ had evaluable cognitive function:
- One child treated at 4.1 years of age retained stable normal cognitive function (performance and language standard scores of 130 and 122, respectively) at age 11.9 years.
- One child treated at 5.6 years of age retained stable normal performance standard score (116 at 11.4 years). While language standard score remained normal (86 at 11.4 years), it declined from 102 at baseline.
ESEJ
Motor and cognitive outcomes for children with ESEJ MLD treated with LENMELDY are presented in Table 6.
Table 6: Motor and Cognitive Outcomes for Children with ESEJ MLD Treated with LENMELDY Patient Age at
Baseline
(Years)Baseline Score*, †, ‡ Age at Last
Assessment
(Years)
Last Assessment Score- *
- Motor = GMFC-MLD score where Level 0 = walking without support quality of performance normal for age; Level 1 = walking without support but with reduced quality of performance; Level 2 = walking with support, walking without support not possible; Level 3 = sitting without support and locomotion such as crawling or rolling, walking without support not possible; Level 4 = sitting without support but no locomotion or sitting without support not possible but locomotion such as crawling or rolling; Level 5 = no locomotion nor sitting without support but head control is possible; Level 6 = loss of any locomotion as well as loss of any head and trunk control.
- †
- Language = Language standard score.
- ‡
- Performance = Performance standard score; obtained on age-appropriate neuropsychological assessments, where normal cognitive function, standard score ≥ 85; mild cognitive impairment, standard score ≥ 70 and < 85; moderate cognitive impairment, standard score >55 and < 70; severe cognitive impairment, standard score ≤ 55.
- §
- Patient 3 and Patient 10 had a mild EJ MLD phenotype at baseline.
- ¶
- Patients 5 and 7 died due to disease progression.
Patient 1 3.2 Motor Level 1
12.7 Motor Level 5
-- 3.2 Language = 103
Performance = 100
7.3 Language = 46
Performance = 56Patient 2 7.3 Motor Level 0
15.5 Motor Level 5
-- 7.3 Language = 110
Performance = 119
15.5 Language = 82
Performance = 87Patient 3§ 11.6 Motor Level 1
19.1 Motor Level 3
-- 11.6 Language = 92
Performance = 119
15.8 Language = 96
Performance = 135Patient 4 7.0 Motor Level 1
14.2 Motor Level 3
-- 7.0 Language = 104
Performance = 89
11.0 Language = 92
Performance = 93Patient 5 5.7 Motor Level 1
6.5 Motor Level 5
-- 5.7 Language = 102
Performance = 82
Died ¶ age 7
years: N/ALanguage/ Performance not assessed
due to patient death.Patient 6 5.5 Motor Level 0
12.5 Motor Level 4
-- 5.5 Language = 118
Performance = 115
9.6
(attempted)Unable to assess after 7.4 years due
to disease progression.Patient 7 5.9 Motor Level 1
6.1 Motor Level 2
-- 5.9 Language = 112
Performance = 87
Died ¶ age 6.6
years: N/ALanguage/ Performance not assessed
due to patient death.Patient 8 4.5 Motor Level 0
7.0 Motor Level 0
-- 4.5 Language = 122
Performance = 129
6.5 Language = 110
Performance = 128Patient 9 2.5 Motor Level 0
5.1 Motor Level 1
-- 2.5 Language = 94
Performance = 120
4.6 Language = 78
Performance = 89Patient 10§ 7.6 Motor Level 0
16.5 Motor Level 2
-- 7.5 Language = 97
Performance = 87
14.3 Language = 80
Performance = 95Note: Age at baseline is the age of the child at the last assessment prior to the time of treatment with LENMELDY.
Four children (Patients 2, 3, 4, and 10) with ESEJ MLD had favorable cognitive outcomes after treatment in the setting of motor decline. Retention of cognitive functioning has not been reported in this phase of EJ MLD disease, as motor and cognitive functioning typically decline together in untreated children.
Gallbladder Disease in Children with PSLI, PSEJ, and ESEJ Treated with LENMELDY
Of the 28 children who had pre-existing gallbladder disease, 14 (50%) had persistent MLD gallbladder disease (regardless of disease subtype) following treatment with LENMELDY. Five children treated with LENMELDY developed gallbladder disease after treatment (regardless of disease subtype).
-
15 REFERENCES
1Kehrer C, Elgün S, Raabe C, Böhringer J, Beck-Wödl S, Bevot A, et al. Association of age at onset and first symptoms with disease progression in patients with metachromatic leukodystrophy. Neurology. 2021;96(2):e255-66.
2Fumagalli F, Zambon AA, Rancoita PMV, Baldoli C, Canale S, Spiga I, et al. Metachromatic leukodystrophy: a single-center longitudinal study of 45 patients. J Inherit Metab Dis. 2021;1-14.
3MacFaul R, Cavanagh N, Lake BD, Stephens R, Whitefield AE. Metachromatic leukodystrophy: review of 38 cases. Arch Dis Child. 1982;57:168-175.
4Kehrer C, Groeschel S, Kustermann-Kuhn B, Bürger F, Köhler W, Kohlschütter A, et al. Language and cognition in children with metachromatic leukodystrophy: onset and natural course in a nationwide cohort. Orphanet J Rare Dis. 2014;9:18.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
LENMELDY is supplied in up to eight infusion bags containing a frozen suspension of genetically modified autologous cells enriched for CD34 +cells. Each bag contains 10 to 20 mL of suspension. Each infusion bag is individually packed within an overwrap in a metal cassette. LENMELDY is shipped from the manufacturing facility to the treatment center storage facility in one to two cryoshipper(s), which may contain multiple metal cassettes intended for a single child. Two shippers would be used in the event that 5-8 bags are manufactured. A Lot Information Sheet is affixed inside the shipper.
- 50 mL infusion bag, overwrap, and metal cassette (NDC 83222-0200-1).
Match the identity of the child with the patient identifiers on the metal cassette(s), infusion bag(s), and Lot Information Sheet upon receipt.
- Store LENMELDY in the vapor phase of liquid nitrogen at less than -130°C (-202°F) until ready for thaw and administration.
- Thaw LENMELDY prior to infusion [ see Dosage and Administration (2)].
- Do not re-freeze after thawing.
- Do not irradiate LENMELDY, as this could lead to inactivation.
- 50 mL infusion bag, overwrap, and metal cassette (NDC 83222-0200-1).
-
17 PATIENT COUNSELING INFORMATION
Ensure that patients and/or caregivers understand the risk of manufacturing failure. A collection of unmanipulated back-up CD34 +cells is required in case of manufacturing failure. These cells must be collected from the patient and be cryopreserved prior to myeloablative conditioning [ see Preparation before LENMELDY Infusion (2.2)].
Prior to treatment, advise patients and/or caregivers of the following:
- Risks associated with mobilization and myeloablative conditioning agents [ see Preparation before LENMELDY Infusion (2.2), Use in Specific Populations( 8.1, 8.3) ].
-
Risk of Hypersensitivity Reactions - Although no cases have been reported to date, allergic reactions may occur with the infusion of LENMELDY. The dimethyl sulfoxide (DMSO) in LENMELDY may cause hypersensitivity reactions, including anaphylaxis [
see Warnings and Precautions (5.4)].
After treatment, advise patients and/or caregivers of the following:
- Thrombosis and Thrombotic Event-A risk of blood clots may occur. Monitor patients for signs and symptoms of thrombosis [ see Warnings and Precautions (5.1)].
- Encephalitis- Monitor patients for signs or symptoms of encephalitis, including neurological deterioration such as weakness, decreased muscle tone, cognitive deterioration, behavioral problems, vomiting, and swallowing difficulties [ see Warnings and Precautions (5.2)].
- Serious Infection- Life-threatening bacterial and viral infections may occur. Monitor patients for signs and symptoms of infection [ see Warnings and Precautions (5.3)].
- Veno-occlusive Disease- Blood sampling will occur before and after LENMELDY infusion to monitor for liver problems including severe, life threatening, veno-occlusive disease [ see Warnings and Precautions (5.4)].
- Delayed Platelet Engraftment- A risk of bleeding exists after myeloablative conditioning and before platelet engraftment and may continue after engraftment in patients who have continued thrombocytopenia [ see Warnings and Precautions (5.5)].
- Neutrophil Engraftment Failure- There is a potential risk of neutrophil engraftment failure and the need for rescue treatment with their back-up collection of CD34 +cells [ see Warnings and Precautions (5.6)].
- Insertional Oncogenesis-There is a potential risk of insertional oncogenesis after treatment with LENMELDY. Patients should be monitored lifelong. Monitoring will include assessment for hematologic malignancies annually for at least 15 years after treatment with LENMELDY. This will include integration site analysis as warranted [ see Warnings and Precautions (5.7)].
Advise patients and/or caregivers to seek immediate attention for the following:
- Signs of a blood clot, which may include pain, discoloration, or swelling of an arm, legs or feet, with warmth over the affected area, unexplained shortness of breath, acute chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body [ see Warnings and Precautions (5.1)].
- New or worsening bleeding or bruising. Platelet recovery following LENMELDY infusion could be delayed, potentially resulting in an increased risk of bruising or bleeding until platelet recovery has been achieved [ see Warnings and Precautions (5.5)].
Advise patients and/or caregivers to:
- Monitor for signs and symptoms of bleeding and have frequent blood draws for platelet counts, until platelet recovery has been achieved [ see Warnings and Precautions (5.5)].
- Have their treating physician contact Orchard Therapeutics at 1-888-878-0185 if they are diagnosed with a malignancy [ see Warnings and Precautions (5.7)] or a blood clot [ see Warnings and Precautions (5.3)].
Advise patients and/or caregivers that patients should not donate blood, organs, tissues, or cells at any time in the future [ see Dosage and Administration (2.3)].
Advise patients and/or caregivers that treatment with LENMELDY may cause a false-positive human immunodeficiency virus (HIV) test result if tested using a PCR assay [ see Warnings and Precautions (5.10)].
Manufactured for:
Orchard Therapeutics North America
Boston, MA 02210
US License No 2263Lenmeldy is a trademark of Orchard Therapeutics plc.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 83222-0200-1
atidarsagene autotemcel
lenmeldy™
Suspension for IV infusion
10 to 20mL containing 1.8 to 11.8 x 106CD34+ cell/mL
For autologous use only.
For intravenous use only. Rx only. Single-dose.
Confirm Patient Identifiers
First Name:______
Last Name:______
Date of Birth: ______ DIN: ______
COI ID: ______ Bag ID: ______
LOT: ______ EXP: ______
Orchard
therapeutics™
Manuf. by: AGC Biologics S.p.A.
20091 Bresso (MI), Italy
Manuf. for: Orchard Therapeutics
Boston, MA 02210
U.S. Lic. 2263
Label P/N: GMP_OTL_331417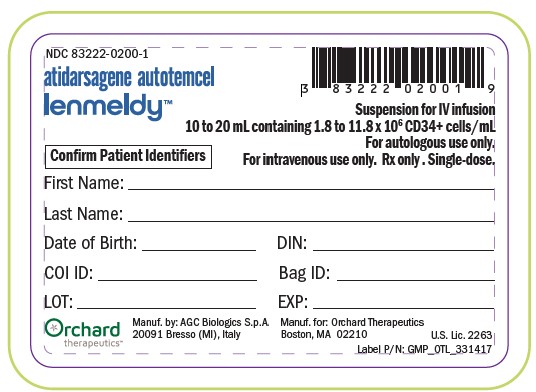
NDC 83222-0200-1
atidarsagene autotemcel
lenmeldy™
Suspension for IV infusion
10 to 20mL containing 1.8 to 11.8 x 106 CD34+ cell/mL
For autologous use only. For intravenous use
only. Rx only. Single-dose.
Confirm Patient Identifiers
Contains genetically modified autologous
hematopoietic stem cells suspended in
cryopreservastion solution containing 5% DMSO.
Store in the vapor phase of liquid nitrogen at
< -130°C until ready for thaw and
administration. Do not unseal the overwrap bag
until after thaw. Once thawed do not re-freeze.
See full prescribing information for dosage and
administration.
Do not use a leukodepleting filter or irradiate.
Not evaluated for infectious substances. No
preservatives.
See Lot Information Sheet for number of
infusion bags and total amount of CD34+ cells.
U.S.Lic. 2263
First Name:______
Last Name:______
Date of Birth: ______
COI ID: ______
DIN: ______
LOT: ______
EXP: ______
Bag ID: ______
Orchard
therapeutics™
Manufactured by:
AGC Biologics S.p.A.
20091 Bresso (MI), Italy
Manufactured for:
Orchard Therapeutics
Boston, MA 02210
Label P/N: GMP_OTL_331413
NDC 83222-0200-1
atidarsagene autotemcel
lenmeldy™
Suspension for IV infusion
10 to 20mL containing 1.8 to 11.8 x 106 CD34+ cell/mL
For autologous use only. For intravenous use
only. Rx only. Single-dose.
Confirm Patient Identifiers
Contains genetically modified autologous
hematopoietic stem cells suspended in
cryopreservastion solution containing 5% DMSO.
Store in the vapor phase of liquid nitrogen at
< -130°C until ready for thaw and
administration. Do not unseal the overwrap bag
until after thaw. Once thawed do not re-freeze.
See full prescribing information for dosage and
administration.
Do not use a leukodepleting filter or irradiate.
Not evaluated for infectious substances. No
preservatives.
See Lot Information Sheet for number of infusion
bags and total amount of CD34+ cells.
No U.S. standard of potency.
U.S.Lic. 2263
First Name:______
Last Name:______
Date of Birth: ______
COI ID: ______
DIN: ______
LOT: ______
EXP: ______
Bag ID: ______
Orchard
therapeutics™
Manufactured by:
AGC Biologics S.p.A.
20091 Bresso (MI), Italy
Manufactured for:
Orchard Therapeutics
Boston, MA 02210
Label P/N: GMP_OTL_331414
-
INGREDIENTS AND APPEARANCE
LENMELDY
atidarsagene autotemcel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:83222-0200 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATIDARSAGENE AUTOTEMCEL (UNII: EPP8G99QG4) (ATIDARSAGENE AUTOTEMCEL - UNII:EPP8G99QG4) ATIDARSAGENE AUTOTEMCEL 11800000 Inactive Ingredients Ingredient Name Strength ISOTONIC SODIUM CHLORIDE SOLUTION (UNII: VR5Y7PDT5W) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83222-0200-1 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125758 04/26/2024 Labeler - Orchard Therapeutics (Europe) Ltd (221097235) Establishment Name Address ID/FEI Business Operations AGC Biologics SPA 428486752 analysis(83222-0200) , api manufacture(83222-0200) , manufacture(83222-0200)