Label: MIPLYFFA- arimoclomol citrate capsule
- NDC Code(s): 72542-124-01, 72542-147-01, 72542-162-01, 72542-193-01
- Packager: Acer Therapeutics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MIPLYFFA - ™safely and effectively. See full prescribing information for MIPLYFFA. MIPLYFFA (arimoclomol) capsules, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMIPLYFFA is indicated for use in combination with miglustat for the treatment of neurological manifestations of Niemann-Pick disease type C (NPC) in adult and pediatric patients 2 years of age and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended oral dosage of MIPLYFFA, in combination with miglustat, for patients with an actual body weight of: 8 kg to 15 kg, is 47 mg three times a day - > 15 kg to ...
-
3 DOSAGE FORMS AND STRENGTHSMIPLYFFA (arimoclomol) capsules are available as follows: MIPLYFFA - StrengthDescription of Capsules - 47 mgWhite opaque body with black printing “47”, and green opaque cap with black ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions such as urticaria and angioedema have been reported in patients treated with MIPLYFFA during Trial 1 - [see Clinical Studies ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described below and elsewhere in the labeling: Hypersensitivity Reactions - [see Warning and Precautions ( 5.1)] ...
-
7 DRUG INTERACTIONS7.1 Effect of MIPLYFFA on Other Drugs - Arimoclomol is an inhibitor of the organic cationic transporter 2 (OCT2) transporter and may increase the exposure of drugs that are OCT2 substrates ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, MIPLYFFA may cause embryofetal harm when administered during pregnancy. There are no available data on ...
-
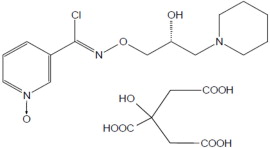
11 DESCRIPTIONMIPLYFFA capsules contain arimoclomol citrate. Arimoclomol citrate is a crystalline powder of white to off-white color that is freely soluble in water. The chemical name is - N-[(2R ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism(s) by which arimoclomol exerts its clinical effects in patients with NPC is unknown. 12.2 Pharmacodynamics - The effect of arimoclomol (744 mg/day for ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year carcinogenicity study in Han Wistar rats, and a 26-week carcinogenicity study in Transgenic rasH2 mice ...
-
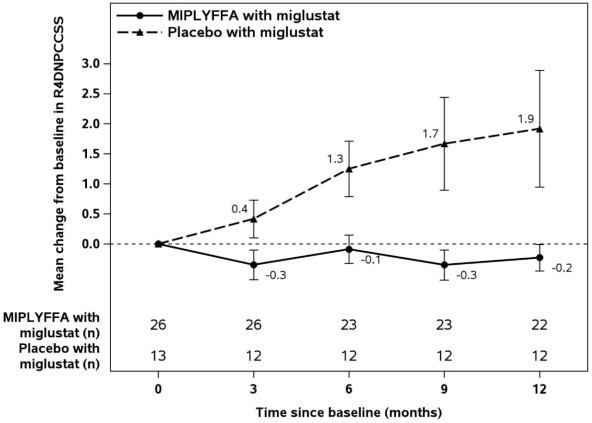
14 CLINICAL STUDIESSafety and effectiveness of MIPLYFFA were assessed in Trial 1, a randomized, double-blind, placebocontrolled, 12-month trial in patients 2 to 19 years of age who had a molecularly confirmed ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - MIPLYFFA (arimoclomol) capsules are supplied in high-density polyethylene (HDPE) bottles with child-resistant closure as follows: MIPLYFFA - StrengthCapsules ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-approved patient labeling ( Instructions for Use). Drug Interactions - Advise the patient to inform his/her healthcare provider if ...
-
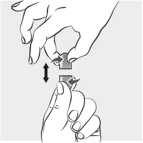
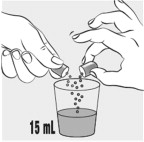
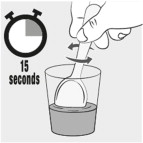
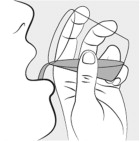
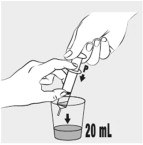
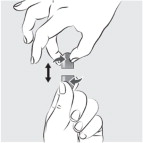
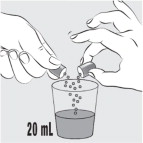
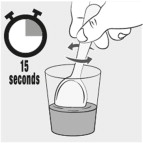
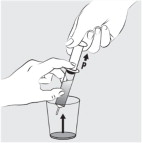
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - MIPLYFFA™ [mye plye' fah] (arimoclomol) capsules for oral use - This Instructions for Use contains information on how to take or give MIPLYFFA. Read this Instructions for ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 47 mg Carton Label - NDC 72542-147-01 - Rx Only - MIPLYFFA™ (arimoclomol) capsules - 47 mg - 90 capsules - For Oral Use
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 62 mg Carton Label - NDC 72542-162-01 - Rx Only - MIPLYFFA™ (arimoclomol) capsules - 62 mg - 90 capsules - For Oral Use
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 93 mg Carton Label - NDC 72542-193-01 - Rx Only - MIPLYFFA™ (arimoclomol) capsules - 93 mg - 90 capsules - For Oral Use
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 124 mg Carton Label - NDC 72542-124-01 - Rx Only - MIPLYFFA™ (arimoclomol) capsules - 124 mg - 90 capsules - For Oral Use
-
INGREDIENTS AND APPEARANCEProduct Information