Label: ANTHRASIL (anthrax immune globulin- human liquid
- NDC Code(s): 60492-0249-1, 60492-0249-2
- Packager: Emergent BioSolutions Canada Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 31, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ANTHRASIL® safely and effectively. See full prescribing information for ANTHRASIL. ANTHRASIL [Anthrax Immune Globulin Intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: INTERACTIONS WITH GLUCOSE MONITORING SYSTEMS AND THROMBOSIS
• Maltose in immune globulin products, including ANTHRASIL, may give falsely high blood glucose levels with some blood point-of-care glucose testing systems (for example those based on the GDH-PQQ or glucose-dye-oxidoreductase methods) resulting in inappropriate administration of insulin and life-threatening hypoglycemia. To avoid interference by maltose contained in ANTHRASIL, perform blood glucose measurements in patients receiving ANTHRASIL with a glucose-specific method (monitor and test strips).
• Thrombosis may occur with immune globulin products, including ANTHRASIL. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
• For patients at risk of thrombosis, administer ANTHRASIL at the minimum infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
Close -
1 INDICATIONS AND USAGE ANTHRASIL is an Anthrax Immune Globulin Intravenous (Human) indicated for the treatment of inhalational anthrax in adult and pediatric patients in combination with appropriate antibacterial drugs ...
-
2 DOSAGE AND ADMINISTRATION For intravenous use only. 2.1 Dose - Table 1 ANTHRASIL Dosing Guide and Intravenous Infusion Rate - Patient GroupDoseaStarting Infusion Rate (first 30 minutes)Incremental Infusion Rate ...
-
3 DOSAGE FORMS AND STRENGTHS Each vial of ANTHRASIL contains a minimum potency of ≥60 units per vial.
-
4 CONTRAINDICATIONS • ANTHRASIL is contraindicated in individuals with a history of anaphylaxis or prior severe systemic reaction associated with the parenteral administration of this or other human immune globulin ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity Reactions - Hypersensitivity reactions may occur with ANTHRASIL. Administer ANTHRASIL in a setting where appropriate equipment, medication (including epinephrine) and ...
-
6 ADVERSE REACTIONS The most common adverse reactions to ANTHRASIL observed in >5% of subjects in the healthy volunteer clinical trial were headache, infusion site pain, nausea, infusion site swelling, and back pain ...
-
7 DRUG INTERACTIONS 7.1 Ciprofloxacin and Levofloxacin - Based on animal studies, ANTHRASIL did not interfere with antibiotic therapy. Concomitant administration of ANTHRASIL with levofloxacin or ciprofloxacin in ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no human data to establish the presence or absence of ANTHRASIL associated risk. 8.2 Lactation - Risk Summary - There are no data to assess the ...
-
11 DESCRIPTION ANTHRASIL, Anthrax Immune Globulin Intravenous (Human), is a sterile solution of purified human immune globulin G (IgG) containing polyclonal antibodies that bind the protective antigen (PA ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - The polyclonal immune globulin G in ANTHRASIL is a passive immunizing agent that neutralizes anthrax toxin. ANTHRASIL binds to protective antigen (PA) to prevent PA ...
-
13 NONCLINICAL TOXICOLOGY Immune globulins are normal constituents of the human body. Toxicology studies have not been performed with ANTHRASIL or its components. The evaluation of new treatment options for anthrax using ...
-
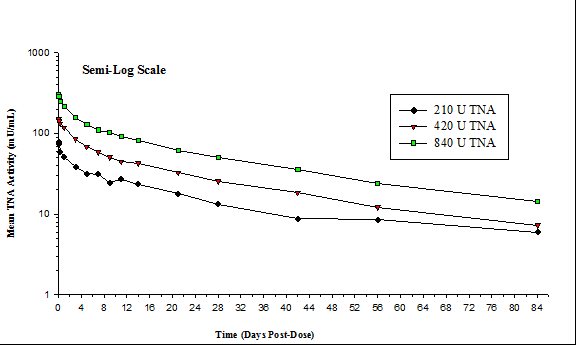
14 CLINICAL STUDIES Because it is not ethical or feasible to conduct placebo-controlled clinical trials in humans with inhalational anthrax, the effectiveness of ANTHRASIL is based on efficacy studies demonstrating a ...
-
15 REFERENCES 1. Kahwaji J, Barker E, Pepkowitz S, Klapper E, Villicana R, Peng A, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - NDC 60492-0249-1 for single vial - NDC 60492-0249-2 for shelf carton containing seven vials - ANTHRASIL is supplied as a 50 mL single dose vial seated with a butyl rubber stopper ...
-
17 PATIENT COUNSELING INFORMATION See FDA-approved patient labeling (Patient Information). Discuss the risks and benefits of this product with the patient or their legally authorized representative before administering it to the ...
-
Patient Information ANTHRASIL [Anthrax Immune Globulin Intravenous (Human)] What is anthrax? Anthrax is a serious disease caused by a germ called Bacillus anthracis. This germ makes a poison called a toxin. People ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

-
Package/Label Display Panel

-
INGREDIENTS AND APPEARANCEProduct Information