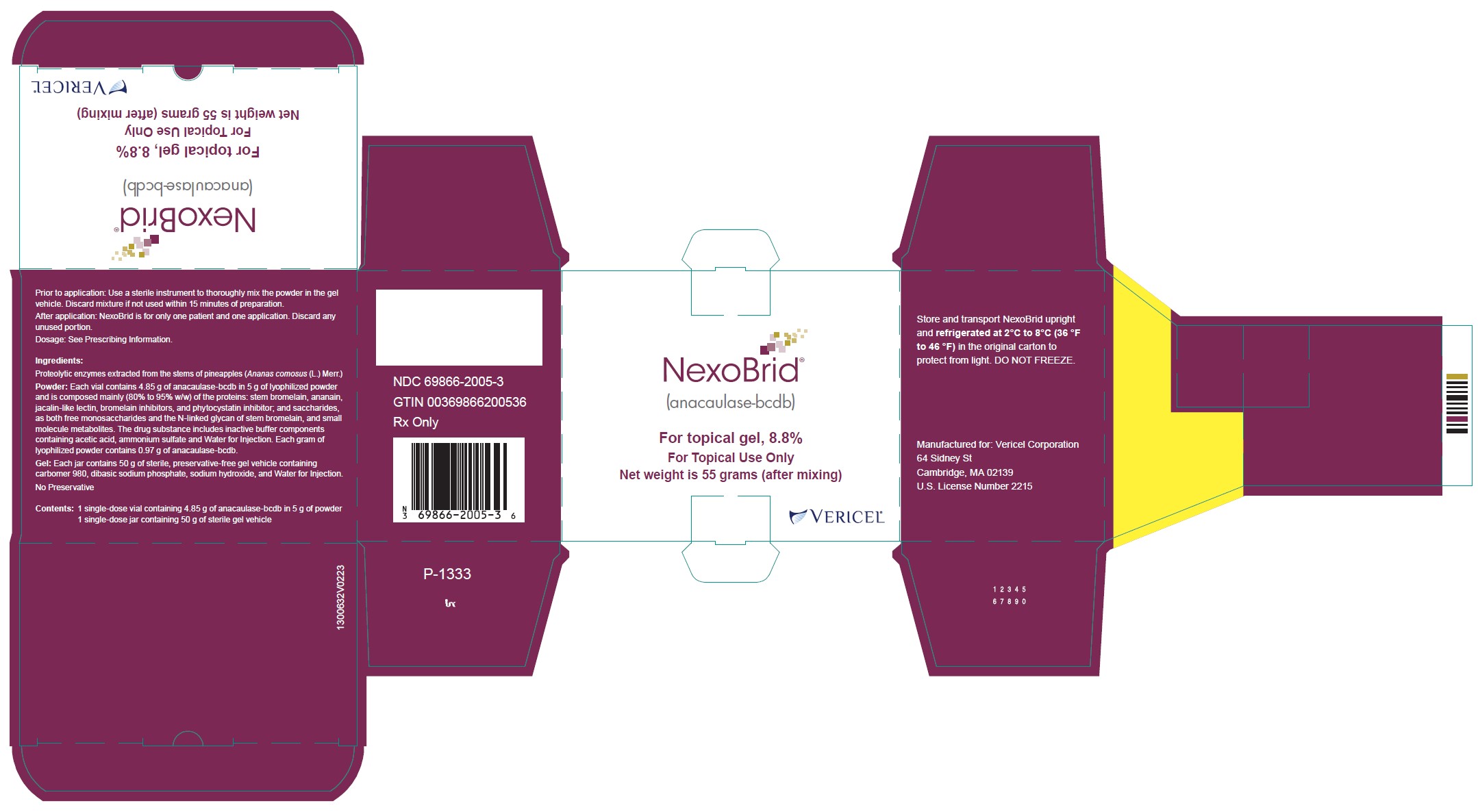
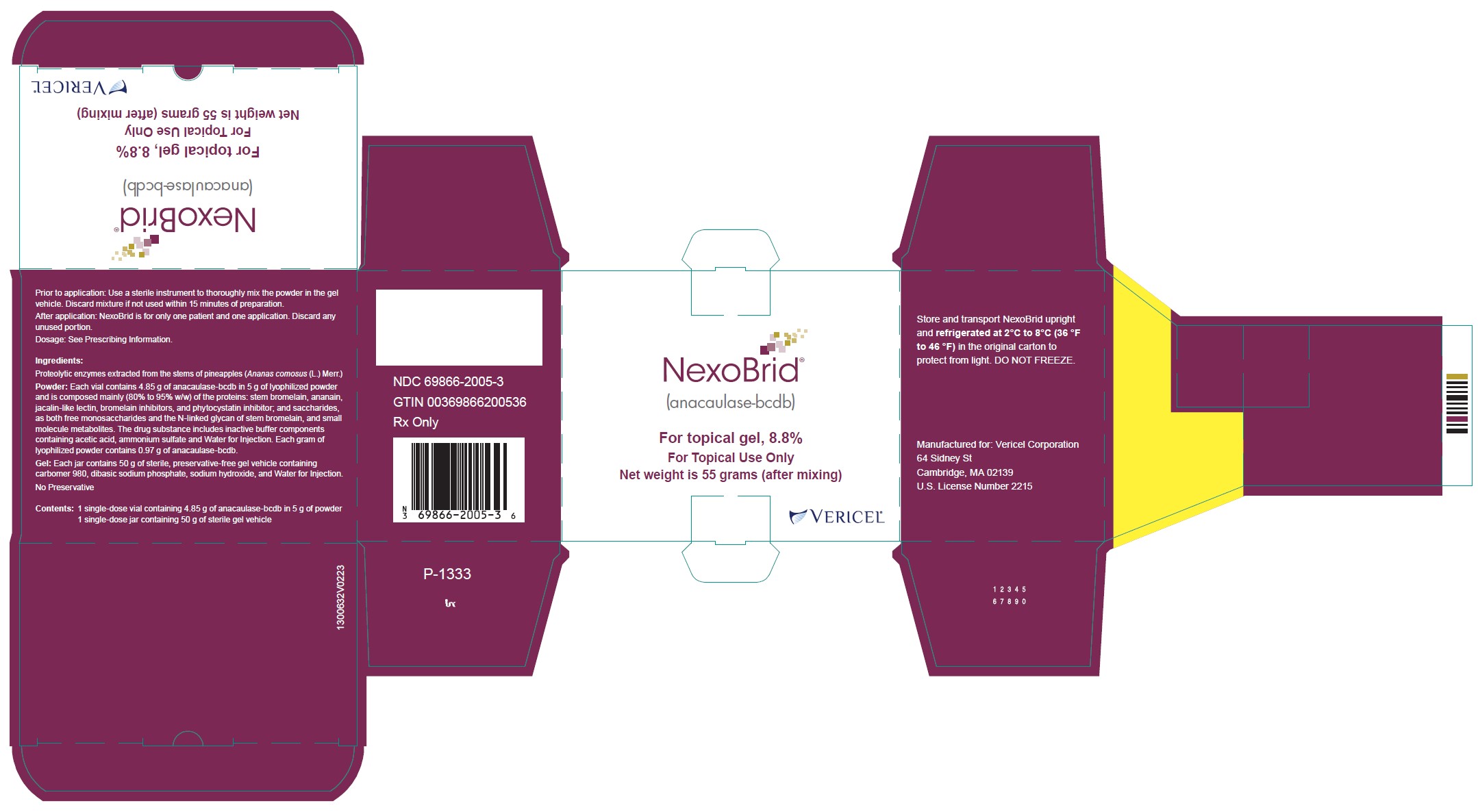
Label: NEXOBRID- anacaulase-bcdb kit
-
NDC Code(s):
69866-2000-1,
69866-2001-2,
69866-2002-3,
69866-2003-1, view more69866-2004-2, 69866-2005-3
- Packager: Vericel Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEXOBRID safely and effectively. See full prescribing information for NEXOBRID.
NEXOBRID® (anacaulase-bcdb), for topical gel
Initial U.S. Approval: 2022INDICATIONS AND USAGE
NEXOBRID contains proteolytic enzymes and is indicated for eschar removal in adults with deep partial thickness and/or full thickness thermal burns (1).
Limitations of Use
The safety and effectiveness of NEXOBRID have not been established for treatment of:
- Chemical or electrical burns (1).
- Burns on the face, perineum, or genitalia (1).
- Burns on the feet of patients with diabetes mellitus or on the feet of patients with occlusive vascular disease (1).
- Circumferential burns (1).
- Burns in patients with significant cardiopulmonary disease, including inhalation injury (1).
NEXOBRID is not recommended for wounds contaminated with radioactive and other hazardous substances to avoid unforeseeable reactions with the product and an increased risk of spreading the noxious substance (1).
DOSAGE AND ADMINISTRATION
For topical use only.
- For topical use only (2.1).
- NEXOBRID may be applied in up to two applications of 4 hours each (2.1, 2.3, 2.5).
- A first application may be applied to an area of up to 15% body surface area (BSA) (2.1).
- A second application may be applied 24 hours later. The total treated area for both applications must not exceed 20% BSA (2.5).
- Use 1.94 g of anacaulase-bcdb in 2 g powder mixed with 20 g gel per 1% BSA, or 4.85 g of anacaulase-bcdb in 5 g powder mixed with 50 g gel per 2.5% BSA (2.1).
- Prepare NEXOBRID at patient’s bedside within 15 minutes of intended application (2.2).
- Apply NEXOBRID to a clean, moist wound bed free of burned epidermis layer and blisters, and cover with an occlusive film dressing for 4 hours (2.2).
- See Full Prescribing Information for additional details on preparation, administration, and removal of NEXOBRID (2).
DOSAGE FORMS AND STRENGTHS
- For topical gel: 8.8% (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarketing use of anacaulase-bcdb. If a hypersensitivity reaction occurs, remove NEXOBRID (if applicable) and initiate appropriate therapy (5.1).
- Pain: Manage pain as appropriate for an extensive dressing change of burn wounds. At least 15 minutes prior to NEXOBRID-related procedures ensure adequate pain control measures are in place (5.2).
- Proteolytic Injury to Non-Target Tissues: NEXOBRID is not recommended for treatment of burn wounds where medical devices or vital structures could become exposed during eschar removal. Protect any open wounds with skin protectant ointments or ointment gauze to prevent possible exposure to NEXOBRID (5.3).
- Coagulopathy: Avoid use of NEXOBRID in patients with uncontrolled disorders of coagulation. Use with caution in patients on anticoagulant therapy or other drugs affecting coagulation, and in patients with low platelet counts and increased risk of bleeding from other causes. Monitor patients for possible signs of coagulation abnormalities and signs of bleeding (5.4).
ADVERSE REACTIONS
The most common adverse reactions (>10%) were pruritus and pyrexia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Vericel Corporation at 1-888-454-BURN or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration Overview
2.2 Preparation of Patient and Burn Wound Treatment Area
2.3 Preparation and Application of NEXOBRID
2.4 Removal of NEXOBRID
2.5 Second Application of NEXOBRID
2.6 Wound Care after Eschar Removal
2.7 Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Pain Management
5.3 Proteolytic Injury to Non-Target Tissues
5.4 Coagulopathy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
NEXOBRID is indicated for eschar removal in adults with deep partial thickness (DPT) and/or full thickness (FT) thermal burns.
Limitations of Use
The safety and effectiveness of NEXOBRID have not been established for treatment of:
- Chemical or electrical burns
- Burns on the face, perineum, or genitalia
- Burns on the feet of patients with diabetes mellitus or on the feet of patients with occlusive vascular disease
- Circumferential burns
- Burns in patients with significant cardiopulmonary disease, including inhalation injury
NEXOBRID is not recommended for wounds contaminated with radioactive and other hazardous substances to avoid unforeseeable reactions with the product and an increased risk of spreading the noxious substance.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration Overview
NEXOBRID is only to be administered by a healthcare provider. Healthcare providers should take precautions to avoid exposure to NEXOBRID during preparation and handling (e.g., use of gloves, surgical masks, other protective coverings, as needed) [see Warnings and Precautions (5.1)].
NEXOBRID lyophilized powder and gel vehicle must be mixed prior to administration. Each vial of lyophilized powder, jar of gel vehicle, and the mixed NEXOBRID are for use for only one patient and for one application [see Dosage and Administration (2.3)].
NEXOBRID is available as:
- 2 g of lyophilized powder (containing 1.94 grams of anacaulase-bcdb) mixed in 20 g gel vehicle per 1% body surface area (BSA), or
- 5 g lyophilized powder (containing 4.85 grams of anacaulase-bcdb) mixed in 50 g gel vehicle per 2.5% BSA.
NEXOBRID is for topical use only.
Apply an ointment skin protectant around the treatment area to create an ointment barrier [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Apply 3 mm thick layer (approximate thickness of a tongue depressor) of NEXOBRID to an area of up to 15% body surface area (BSA) in one application.
If the wound area is more than 15% BSA, apply NEXOBRID in 2 separate sessions (e.g., treat up to 15% BSA in one session and up to 5% BSA in a second session). Apply the second application of NEXOBRID twenty-four (24) hours after the first application to the same or new burn wound area. The total treatment area must not exceed 20% BSA (40 grams of NEXOBRID lyophilized powder) across two treatment sessions [see Dosage and Administration (2.3, 2.4, 2.5)].
2.2 Preparation of Patient and Burn Wound Treatment Area
Use pain management as practiced for an extensive dressing change of burn wounds 15 minutes prior to all NEXOBRID related procedures.
Prepare the wound area as follows:
- Thoroughly clean the wound to remove any charred tissue, blisters, and any topical products.
- Apply a dressing soaked with an antibacterial solution to the treatment area for at least 2 hours.
- Ensure the wound bed is clear of any remnants of topical agents (e.g. silver sulfadiazine or povidone iodine).
- Apply an ointment skin protectant (e.g., petrolatum) 2 to 3 cm outside of the treatment area to create an ointment barrier.
- 5. Protect any open wounds (e.g., laceration, abraded skin and escharotomy incision) with skin protectant ointments or ointment gauze to prevent possible exposure to NEXOBRID [see Warnings and Precautions (5.3)].
- Avoid applying the ointment to the treatment area itself, as this would impede direct contact of NEXOBRID with the eschar.
2.3 Preparation and Application of NEXOBRID
Gather the following supplies prior to NEXOBRID preparation and application. All supplies should be sterile:
- Instrument for mixing (e.g., spatula or tongue depressor)
- Tongue depressor for NEXOBRID application
- 0.9% Sodium Chloride Irrigation
- Occlusive film dressing
- Loose, thick fluffy dressing and bandage
Maintain pain management throughout the application as practiced for an extensive dressing change of burn wounds. At least 15 minutes prior to NEXOBRID application, ensure adequate pain control measures are in place to address NEXOBRID-related pain.
Preparation
Prepare NEXOBRID at the patient’s bedside within 15 minutes of the intended application.
Using aseptic technique, mix NEXOBRID lyophilized powder and gel vehicle as follows:
- Pour the NEXOBRID lyophilized powder into the gel vehicle jar.
- Thoroughly mix the NEXOBRID lyophilized powder and gel vehicle using a sterile instrument (e.g., tongue depressor or spatula) until the mixture is uniform. The mixed lyophilized powder and gel vehicle produce NEXOBRID in a final concentration of 8.8% w/w.
Discard NEXOBRID if not used within 15 minutes of preparation, as the enzymatic activity of the product decreases progressively following mixing.
Application
Apply NEXOBRID within 15 minutes of preparation as follows:
- Moisten the treatment area by sprinkling sterile 0.9% Sodium Chloride Irrigation onto the burn wound.
- Using a sterile tongue depressor, completely cover the moistened treatment area with the mixed NEXOBRID in a 3 mm thick layer (approximate thickness of a tongue depressor) that completely covers the burn wound area.
- Cover the treated wound with a sterile occlusive film dressing.
- Gently press the occlusive film dressing at the area of contact with the ointment barrier to ensure adherence between the occlusive film dressing and the sterile ointment barrier and to achieve complete containment of NEXOBRID on the treatment area. NEXOBRID gel should fill the entire volume of the treatment area, and there should be no visible air under the occlusive film dressing.
- Cover the dressed wound with a sterile loose, thick, fluffy dressing and secure with a sterile bandage.
- Leave the dressing and NEXOBRID in place for 4 hours.
- Discard any unused portions of NEXOBRID.
2.4 Removal of NEXOBRID
Remove NEXOBRID after 4 hours. Gather the following supplies prior to NEXOBRID removal. All supplies should be sterile:
- Blunt-edged instruments (e.g., tongue depressor)
- Large dry gauze
- Gauze soaked with 0.9% Sodium Chloride Irrigation
- Dressing soaked with an antibacterial solution
Implement and maintain pain management as practiced for an extensive dressing change of burn wounds throughout the following removal procedure:
- Remove the occlusive film dressing using aseptic technique.
- Remove the ointment barrier using a sterile blunt-edged instrument.
- Remove the dissolved eschar from the wound by scraping it away with a sterile blunt-edged instrument.
- Wipe the wound thoroughly with a large sterile dry gauze, then wipe with a sterile gauze that has been soaked with sterile 0.9% Sodium Chloride Irrigation. Rub the treated area until the appearance of a clean dermis or subcutaneous tissues with pinpoint bleeding.
- To remove remnants of dissolved eschar, apply a dressing soaked with an antibacterial solution for at least 2 hours.
2.5 Second Application of NEXOBRID
A second application of NEXOBRID may be applied 24 hours following the first application to either the same area previously treated with NEXOBRID or to a new area [see Dosage and Administration (2.2, 2.3,2.4)]. A second application may be considered if:
- The wound area is more than 15% BSA, or
- Multiple wound areas on different body surfaces require two treatments for logistical reasons such as body position, or
- The first application’s eschar removal was not complete.
The total treated area must not exceed 20% BSA, inclusive of both applications [see Dosage and Administration (2.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
NEXOBRID-Treated Patients
Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarketing use of NEXOBRID [see Adverse Reactions (6.2)]. If a hypersensitivity reaction occurs, remove NEXOBRID (if applicable) and initiate appropriate therapy [see Dosage and Administration (2.7)].
NEXOBRID is contraindicated in patients with a known hypersensitivity to anacaulase- bcdb, bromelain, pineapples or to any other component of NEXOBRID. NEXOBRID is also contraindicated in patients with known hypersensitivity to papayas or papain because of the risk of cross-sensitivity.
Healthcare Providers Preparing and Applying NEXOBRID
Healthcare personnel should take appropriate precautions to avoid exposure when preparing and handling NEXOBRID (e.g., gloves, surgical masks, other protective coverings, as needed). In the event of inadvertent skin exposure, rinse NEXOBRID off with water to reduce the likelihood of skin sensitization.
5.2 Pain Management
Eschar removal with NEXOBRID and treatment-related burn wound procedures are painful and require adequate analgesia and/or anesthesia. Pain management should be appropriate for an extensive dressing change of burn wounds. At least 15 minutes prior to NEXOBRID application ensure adequate pain control measures are in place to address NEXOBRID-related pain [see Dosage and Administration (2.2, 2.3, 2.4)].
5.3 Proteolytic Injury to Non-Target Tissues
NEXOBRID is not recommended for treatment of burn wounds where medical devices (e.g., implants, pacemakers, shunts) or vital structures (e.g., large vessels) could become exposed during eschar removal.
Protect any open wounds (e.g., laceration, abraded skin and escharotomy incision) with skin protectant ointments or ointment gauze to prevent possible exposure to NEXOBRID [see Dosage and Administration (2.2)].
5.4 Coagulopathy
A reduction of platelet aggregation and plasma fibrinogen levels and a moderate increase in partial thromboplastin and prothrombin times have been reported in the literature as possible effects following oral administration of bromelain, a component of NEXOBRID. In vitro and animal data suggest that bromelain can also promote fibrinolysis.
Avoid use of NEXOBRID in patients with uncontrolled disorders of coagulation. Use NEXOBRID with caution in patients on anticoagulant therapy or other drugs affecting coagulation, and in patients with low platelet counts and increased risk of bleeding from other causes (e.g., peptic ulcers and sepsis). Patients should be monitored for possible signs of coagulation abnormalities and signs of bleeding.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
Studies 1 and 2 evaluated subjects undergoing eschar removal for deep partial thickness (DPT) and/or full thickness (FT) thermal burns [see Clinical Studies (14)]. An integrated analysis of safety data from Studies 1 and 2 compared NEXOBRID (n=177) to standard of care (SOC) (n=149). The SOC treatment included both surgical and non-surgical eschar removal methods.
The mean age of the safety population was 35.6 years; 73% were male; 81% were White, 9% were Black, 6% were other races, and 3% were Asian. Regarding burn etiology, 65% had fire/flame burns, 26% had scald burns, 8% had contact burns, and <1% had burns of other etiology. The mean body surface area (BSA) of wounds that received study treatment was 9.2±5.07%. Mean BSA of all burn wounds was 12.0±6.05%. Of the 177 subjects who were treated with NEXOBRID in Studies 1 and 2, 159 (90%) received one treatment of NEXOBRID, and 18 (10%) received 2 treatments.
Table 1 presents adverse reactions that occurred in ≥1% of subjects in the NEXOBRID arm and at a higher incidence than the SOC arm, up to 3 months following wound closure.
Table 1: Adverse Reactions Reported in ≥1% and Greater Incidence than Standard of Care in NEXOBRID-Treated Patients for Eschar Removal in Deep Partial Thickness and/or Full Thickness Thermal Burns in Studies 1 and 2a
a During the time period from baseline to 3 months post wound closure b Standard of Care treatment included both surgical and non-surgical eschar removal methods c Wound complication includes skin graft failure, graft loss, graft complication, and wound decomposition NEXOBRID
(N = 177)
Patients
n (%)Standard of Careb
(N = 149)
Patients
n (%)Pruritus 27 (15) 19 (13) Pyrexia 21 (12) 13 (9) Wound complicationc 15 (9) 9 (6.0) Anemia 11 (6) 8 (5) Vomiting 9 (5) 4 (3) Insomnia 8 (5) 6 (4) Urinary tract infection 7 (4) 1 (1) Tachycardia 5 (3) 0 Rash 6 (3) 0 Infection 4 (2) 2 (1) Sepsis 4 (2) 1 (1) Leukocytosis 3 (2) 1 (1) Hypotension 3 (2) 1 (1) Hepatic function abnormal 2 (1) 0 Drug hypersensitivity 2 (1) 0 Bacteremia 2 (1) 0 Scar 2 (1) 0 Subcutaneous hematoma 2 (1) 0 Decubitus ulcer 2 (1) 0 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of anacaulase-bcdb outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Hypersensitivity, including anaphylaxis and urticaria
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on NEXOBRID use in pregnant women to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
In embryofetal developmental studies in rats and rabbits, intravenous doses up to 4 and 0.1 mg/kg/day NEXOBRID were administered to pregnant rats and rabbits, respectively, during organogenesis. No significant developmental toxicities were observed in these studies. However, severe maternal toxicities were noted and the tolerable maternal exposure levels were much lower compared with the maximum human exposure in clinical setting.
8.2 Lactation
Risk Summary
There are no data on the presence of anacaulase-bcdb in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NEXOBRID and any potential adverse effects on the breastfed infant from NEXOBRID or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of NEXOBRID in pediatric patients have not been established.
8.5 Geriatric Use
Of the 177 subjects exposed to NEXOBRID for eschar removal in deep partial thickness (DPT) and/or full thickness (FT) thermal burns, 6 (3%) subjects were 65 years or older, and 1 (< 1%) subject was 75 years or older [see Clinical Studies (14)]. Clinical studies of NEXOBRID did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
-
11 DESCRIPTION
The drug substance in NEXOBRID, anacaulase-bcdb, is a mixture of proteolytic enzymes extracted from the stems of pineapple plants (Ananas comosus [L.] Merr.) that has been sterile filtered and lyophilized. The drug substance, anacaulase-bcdb, is composed mainly (80% to 95% w/w) of the proteins: stem bromelain, ananain, jacalin-like lectin, bromelain inhibitors, and phytocystatin inhibitor; and saccharides, as both free monosaccharides and the N-linked glycan of stem bromelain, and small molecule metabolites. The drug substance includes inactive buffer components containing acetic acid, ammonium sulfate, and Water for Injection. Each gram of lyophilized powder contains 0.97 grams of anacaulase-bcdb.
NEXOBRID (anacaulase-bcdb) for topical gel is a botanical drug product supplied as a sterile, preservative-free, lyophilized powder in a single-dose glass vial that must be mixed in a gel vehicle supplied in a single-dose glass jar prior to application. Mixture of either 2 grams of lyophilized powder (containing 1.94 grams of anacaulase-bcdb) or 5 grams of lyophilized powder (containing 4.85 grams of anacaulase-bcdb) in 20 grams or 50 grams of gel vehicle, respectively, provides an 8.8% w/w, yellowish white to light brown opaque gel for topical use. The pH of the topical gel mixture is approximately 6.2 to 6.7.
The co-packaged 20 gram or 50 gram jar of the sterile, preservative-free gel vehicle contains carbomer 980, dibasic sodium phosphate, sodium hydroxide, and Water for Injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mixture of enzymes in NEXOBRID dissolves burn wound eschar. The specific components responsible for this effect have not been identified.
12.2 Pharmacodynamics
The pharmacodynamics of NEXOBRID are unknown.
Cardiac Electrophysiology
At the approved recommended dose, NEXOBRID did not prolong the QT interval in humans to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Topically applied NEXOBRID to deep partial and full thickness burn wounds is rapidly absorbed, with median serum Tmax of 4 hours (during the treatment application). Systemic exposure (i.e., AUC) of bromelain, a component of anacaulase-bcdb is correlated with the size of the treated area and NEXOBRID dose, but not the depth of the burn wound.
Elimination
A majority of subjects had no quantifiable serum concentrations after 72 hours. The mean ± SD terminal half-life of bromelain, a component of anacaulase-bcdb, is 12 ± 4.4 hours.
Cmax and the dose‑normalized Cmax values after the first and second application (mean dosing interval of 17 hours) are comparable and only slight accumulation (less than 2-fold difference) is seen in AUC0-4 and AUC0-4 dose‑normalized levels after the second application, compared to the first application.
Drug Interaction Studies
Effect of NEXOBRID on Other Drugs
Bromelain, a component of anacaulase-bcdb, exhibited CYP2C8 time-dependent inhibition in human hepatocytes and inhibited human microsomal CYP2C9. No clinical studies have been conducted to assess the potential for systemic drug interactions.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of NEXOBRID for the eschar removal of deep partial thickness (DPT) and full thickness (FT) thermal burns has been investigated in two trials.
Study 1
NEXOBRID was investigated in the DETECT randomized, controlled, assessor-blinded, three-arm study, comparing NEXOBRID, standard of care (SOC), and gel vehicle treatment in subjects with DPT and/or FT thermal burns of 3 - 30% BSA (Study 1, NCT02148705). SOC included both surgical and non-surgical methods for eschar removal per the investigators’ discretion. Subjects on the NEXOBRID and gel vehicle arms who had eschar remaining following the topical treatment period were treated with SOC. NEXOBRID was compared to gel vehicle for the incidence of ≥95% eschar removal at the end of the topical treatment period. NEXOBRID was also compared with SOC for the incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) and time to eschar removal.
A total of 175 subjects were randomized in a 3:3:1 ratio (NEXOBRID : SOC : gel vehicle) and 169 subjects were treated. The mean age was 41 years, 70% of subjects were male and 30% were female, and 81% were White, 14% were Black or African American, 5% were other races, and 1% were Asian. Seventeen percent of subjects were Hispanic or Latino. Subjects had one or more target wounds (TWs) to be treated for eschar removal. The mean percentage BSA of all TWs per subject was 6.1%. The majority of subjects (82%) had one to two TWs.
The incidence of ≥95% eschar removal at the end of the topical treatment period for subjects in the NEXOBRID and gel vehicle groups is shown in Table 2.
Table 2: Incidence of ≥95% Eschar Removal at the End of the Topical Treatment Period in NEXOBRID- or Gel Vehicle-Treated Subjects with Deep Partial Thickness and/or Full Thickness Thermal Burns (Study 1; DETECT)
NEXOBRID
(N=75)Gel Vehicle
(N=25)Treatment Difference
(95% Confidence Interval)93%
(70/75)4%
(1/25)89% (74%, 96%) The incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) and time to ≥95% eschar removal for the NEXOBRID and SOC groups are shown in Table 3.
Table 3: Incidence of Excision for Eschar Removal in NEXOBRID- or SOC-Treated Subjects with Deep Partial Thickness and/or Full Thickness Thermal Burns (Study 1; DETECT)
SOC = standard of care NEXOBRID
(N=75)SOC
(N=75)Treatment Difference
(95% Confidence Interval)4%
(3/75)72%
(54/75)-68% (-78%, -56%) The median time to eschar removal was 1 day on the NEXOBRID arm and 3.8 days on the SOC arm.
The estimated median time to ≥95% wound closure for all TWs on a subject was 31 days for the NEXOBRID arm and 36 days for the SOC treatment arm. Subjects were not evaluated frequently enough after achieving ≥95% wound closure to adequately assess time to 100% wound closure.
Study 2
NEXOBRID was investigated in a multicenter, open-label, randomized, two-arm study, comparing NEXOBRID to SOC treatment in subjects with DPT and/or FT thermal burns of 5 - 24% BSA (Study 2; NCT00324311). SOC included both surgical and non-surgical methods for eschar removal per the investigators’ discretion. The study enrolled 182 subjects. The first subject at each site (26 subjects) was not randomized and was treated with NEXOBRID. The remaining 156 subjects were randomized to NEXOBRID or SOC. The efficacy assessments were analyzed on DPT burns only.
Demographics were similar across both arms. The mean age was 29.9 years. Approximately 80% of the study subjects were adults (≥18 years), 74% were male and 26% were female, 82% were White, 7% were other races, 6% were Black, and 5% were Asian.
The incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) for the NEXOBRID and SOC groups is shown in Table 4.
Table 4: Incidence of Excision of Eschar Removal of Deep Partial Thickness Wounds in Patients with Thermal Burns (Study 2)
SOC = standard of care
aAnalysis population includes only patients with at least one wound that was entirely DPT
bSurgical eschar removal procedures include (tangential, minor, avulsion, Versajet and/or dermabrasion excision)
cAssessement per subject was an exploratory analysisNEXOBRID
N=106 Wounds
in 49 SubjectsaSOC
N=88 Wounds
in 48 SubjectsaTreatment Difference
(95% Confidence Interval)Incidence of excision for
eschar removal
(per wound)b15%
16/106 wounds63%
55/88 wounds-47% (-59%, -34%) Incidence of excision for
eschar removal
(per subject)b, c22%
11/49 subjects77%
37/48 subjects-55% (-71%, -38%) In randomized subjects, the estimated median time to ≥95% wound closure was 33 days for the NEXOBRID arm and 24 days for the SOC treatment arm. Subjects were not evaluated frequently enough after achieving ≥95% wound closure to adequately assess time to 100% wound closure.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NEXOBRID (anacaulase-bcdb) for topical gel, 8.8%, is supplied as a package containing two components, a sterile, preservative-free, off-white to light tan lyophilized powder in a glass vial and a sterile, preservative-free, clear and colorless gel vehicle in a glass jar, that are mixed prior to application [see Dosage and Administration (2.3)].
NEXOBRID is available:
- One glass vial of 2 g lyophilized powder (containing 1.94 grams of anacaulase-bcdb) and one glass jar of 20 g gel vehicle per carton (NDC 69866-2002-3)
- One glass vial of 5 g lyophilized powder (containing 4.85 grams of anacaulase-bcdb) and one glass jar of 50 g gel vehicle per carton (NDC 69866-2005-3)
Storage and Handling
Store and transport NEXOBRID package upright and refrigerated at 2℃ to 8℃ (36 ℉ to 46 ℉) in the original carton to protect from light. DO NOT FREEZE.
Do not use if the vial or jar are damaged [see Dosage and Administration (2.3)].
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients of the risk of hypersensitivity reactions, including anaphylaxis [see Warnings and Precautions (5.1)].
Manufactured for:
Vericel Corporation
64 Sidney Street,
Cambridge, MA 02139
U.S. License number 2215
NEXOBRID® is a registered trademark of MediWound LTD.
L65629.3
- PRINCIPAL DISPLAY PANEL - NDC: 69866-2002-3 - 2g/20g Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-2005-3 - 5g/50g Carton Label
-
INGREDIENTS AND APPEARANCE
NEXOBRID
anacaulase-bcdb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69866-2002 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2002-3 1 in 1 CARTON 12/29/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 2 g Part 2 1 JAR 20 g Part 1 of 2 NEXOBRID
anacaulase-bcdb powderProduct Information Item Code (Source) NDC:69866-2000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANACAULASE-BCDB (UNII: JD24M53P9U) (ANACAULASE-BCDB - UNII:JD24M53P9U) ANACAULASE-BCDB 158 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) AMMONIUM SULFATE (UNII: SU46BAM238) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2000-1 2 g in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Part 2 of 2 STERILE
sterile gelProduct Information Item Code (Source) NDC:69866-2001 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2001-2 20 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 NEXOBRID
anacaulase-bcdb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69866-2005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2005-3 1 in 1 CARTON 12/29/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 g Part 2 1 JAR 50 g Part 1 of 2 NEXOBRID
anacaulase-bcdb powderProduct Information Item Code (Source) NDC:69866-2003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANACAULASE-BCDB (UNII: JD24M53P9U) (ANACAULASE-BCDB - UNII:JD24M53P9U) ANACAULASE-BCDB 158 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) AMMONIUM SULFATE (UNII: SU46BAM238) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2003-1 5 g in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Part 2 of 2 STERILE
sterile gelProduct Information Item Code (Source) NDC:69866-2004 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-2004-2 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Labeler - Vericel Corporation (079745570)