Label: TECELRA- afamitresgene autoleucel injection, suspension
- NDC Code(s): 83205-0001-2
- Packager: Adaptimmune LLC
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use TECELRA safely and effectively. See full prescribing information for TECELRA ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME
Cytokine Release Syndrome (CRS), which may be severe or life-threatening, occurred in patients receiving TECELRA. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care. Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage CRS [see Preparation and Administration (2.2), and Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGETECELRA is a melanoma-associated antigen A4-(MAGE-A4)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adults with unresectable or metastatic synovial ...
-
2 DOSAGE AND ADMINISTRATIONFor autologous use only. For intravenous use only. 2.1 Recommended Dose - The recommended dose is between 2.68 x 109 to 10 x 109 MAGE-A4 T cell receptor (TCR) positive T cells administered as a ...
-
3 DOSAGE FORMS AND STRENGTHSTECELRA is a cell suspension for intravenous infusion. A single dose of TECELRA contains 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells in one or more infusion bag(s) [see How ...
-
4 CONTRAINDICATIONSDO NOT use TECELRA in adults who are heterozygous or homozygous for HLA- A*02:05P .
-
5 WARNINGS AND PRECAUTIONS5.1 Cytokine Release Syndrome - Cytokine release syndrome (CRS), including potentially life-threatening reaction has been observed following administration of TECELRA. CRS occurred in 75% of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNone
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with TECELRA use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with TECELRA to assess ...
-
11 DESCRIPTIONTECELRA (afamitresgene autoleucel) is a melanoma-associated antigen A4 (MAGE- A4)-directed genetically modified autologous T cell immunotherapy product consisting of CD4 and CD8 positive T cells ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - TECELRA is a genetically modified autologous T cell immunotherapy consisting of CD4 and CD8 positive T cells transduced with a self-inactivating LV to express an ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with TECELRA. A genomic insertion site analysis was performed on TECELRA ...
-
14 CLINICAL STUDIESLocally Inoperable/ Metastatic Synovial Sarcoma - The efficacy of TECELRA was evaluated in a multicenter, single-arm, open-label clinical trial (SPEARHEAD-1, Cohort 1). The study enrolled ...
-
15 REFERENCESLee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019; 25 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTECELRA is supplied in one or more infusion bag(s) containing a frozen suspension of genetically modified autologous T cells in 5% DMSO. Each TECELRA infusion bag is individually packed in a metal ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Discuss the following with the patient: Inform patients that there is a chance of manufacturing or delivery ...
-
MEDICATION GUIDEMEDICATION GUIDE - TECELRA® (pronounced tuh-sel-ruh) (afamitresgene autoleucel) Issued: Aug 2024 - Read this Medication Guide before you start your TECELRA treatment. Talk with your ...
-
PRINCIPAL DISPLAY PANEL - Bag and Cassette LabelNDC 83205-0001-2 - afamitresgene autoleucel - Tecelra® Rx ONLY - FOR AUTOLOGOUS & INTRAVENOUS USE ONLY - No U.S. standard of potency - Contains: 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells ...
-
PRINCIPAL DISPLAY PANEL - Bag Label - Patient Identifierafamitresgene autoleucel - Tecelra® VERIFY PATIENT - IDENTIFIERS - Patient MRN: MAX14CHARACTER - COID: MAX.18-CHARACTERS/ First Name MI: MAX.14CHARACTR - Last Name: MAX-14CHARACTR - DOB: 01-Jan-2000 - DIN ...
-
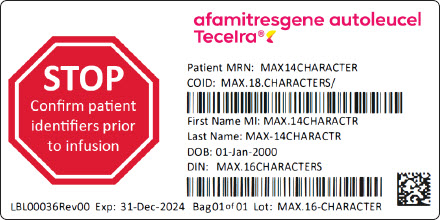
PRINCIPAL DISPLAY PANEL - Cassette Label - Patient IdentifierSTOP - Confirm patient - identifiers prior - to infusion - afamitresgene autoleucel - Tecelra® Patient MRN: MAX14CHARACTER - COID: MAX.18.CHARACTERS/ First Name MI: MAX.14CHARACTR - Last Name ...
-
INGREDIENTS AND APPEARANCEProduct Information