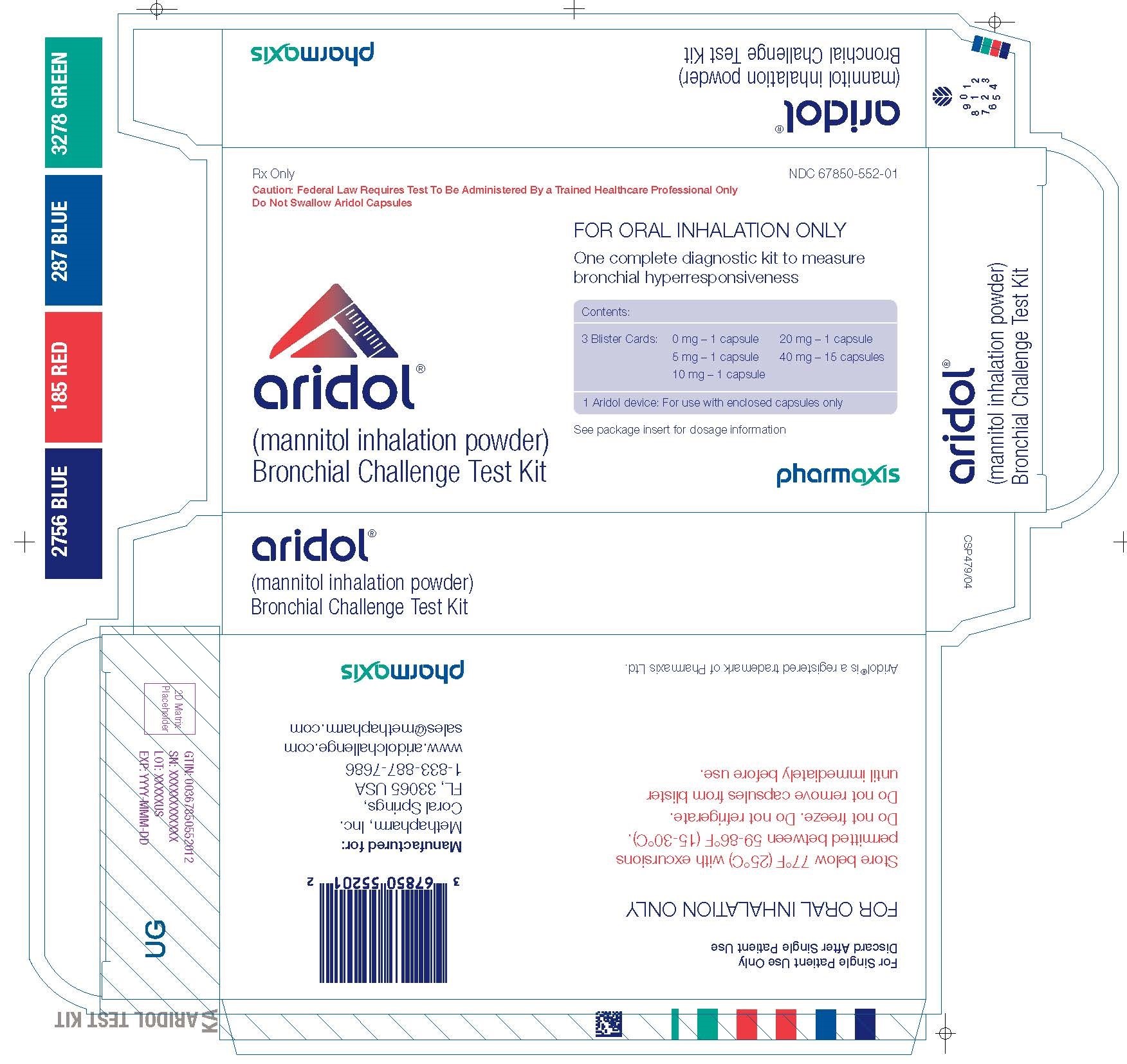
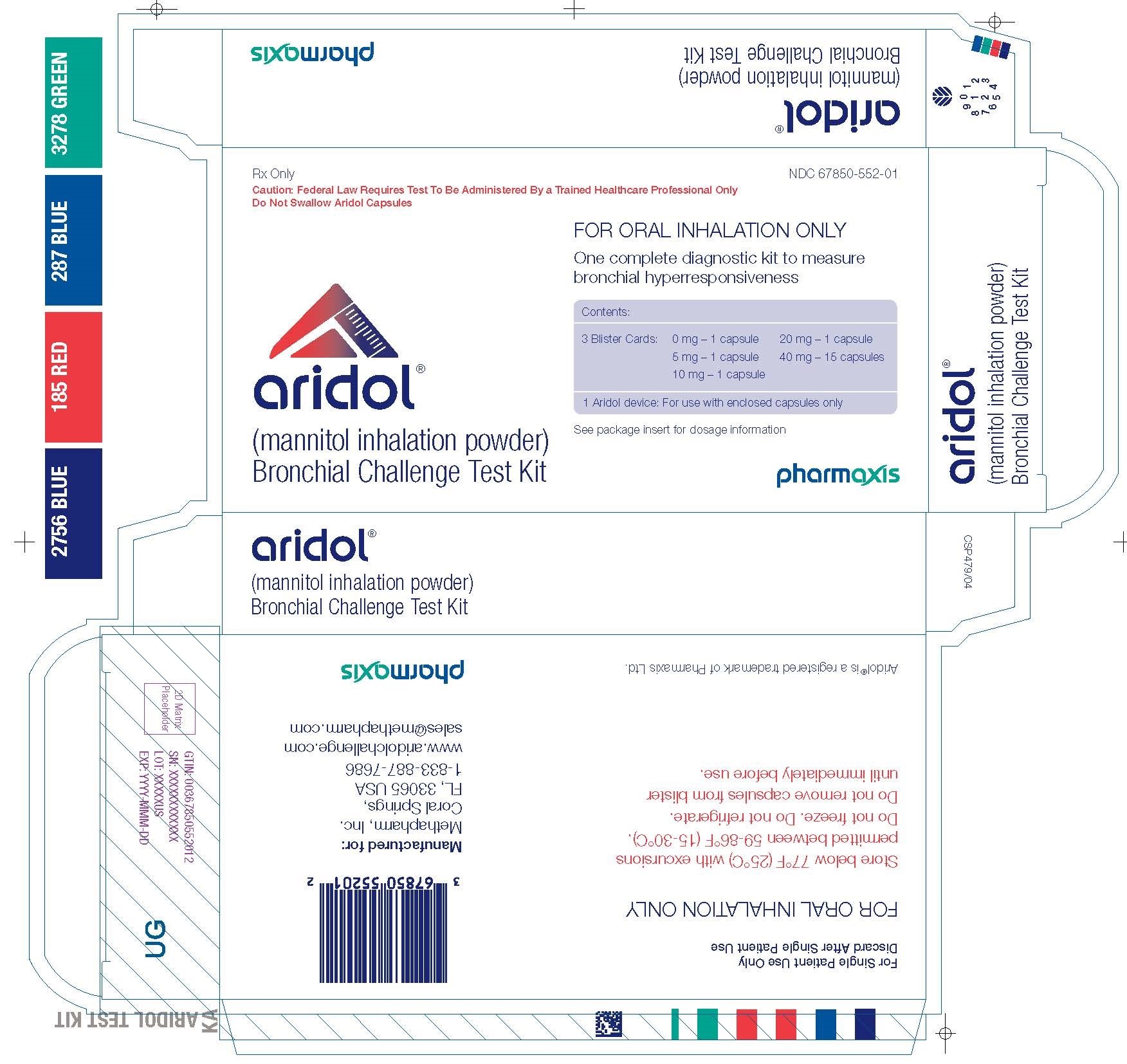
Label: ARIDOL BRONCHIAL CHALLENGE TEST KIT- mannitol kit
- NDC Code(s): 67850-552-01
- Packager: Methapharm, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ARIDOL safely and effectively. See full prescribing information for ARIDOL.
ARIDOL® (mannitol inhalation powder) , for oral inhalation use
Bronchial Challenge Test Kit
Initial U.S. Approval: 1964WARNING: RISK OF SEVERE BRONCHOSPASM
See full prescribing information for complete boxed warning.
Mannitol, the active ingredient in ARIDOL, acts as a bronchoconstrictor and may cause severe bronchospasm. Bronchial challenge testing with ARIDOL is for diagnostic purposes only. Only trained professionals under the supervision of a physician who are familiar with the management of acute bronchospasm should perform bronchial challenge testing with ARIDOL. Medications (such as short-acting inhaled beta-agonist) and equipment to treat severe bronchospasm must be present in the testing area. Because of the potential for severe bronchoconstriction, bronchial challenge testing with ARIDOL should not be performed in any patient with clinically apparent asthma or very low baseline pulmonary function tests (e.g., FEV1<1-1.5 liters or <70% of the predicted values) (5.1)
INDICATIONS AND USAGE
ARIDOL is a sugar alcohol indicated for the assessment of bronchial hyperresponsiveness in adult and pediatric patients 6 years of age or older who do not have clinically apparent asthma. (1)
Limitations of Use: ARIDOL is not a standalone test or a screening test for asthma. Bronchial challenge testing with ARIDOL should be used only as part of a physician's overall assessment of asthma.
DOSAGE AND ADMINISTRATION
For Oral Inhalation Use Only
- One ARIDOL test kit contains dry powder mannitol capsules in graduated doses and a single patient use inhaler necessary to perform one bronchial challenge test. (2)
- The mannitol capsules supplied in the ARIDOL kit are to be used with the single patient use inhaler device (2). Discard the inhaler after use.
- Capsule contents are to be inhaled in increasing dosage until either a positive response (15% reduction in FEV1 from baseline or a 10% incremental reduction in FEV1 between consecutive doses) is achieved or all capsules are inhaled (maximum total dose 635mg) (2)
- Starting and maximum dose is the same for children (≥6 years old) and adults (2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Severe bronchospasm: ARIDOL may cause severe bronchospasm in susceptible patients. Administer by trained professionals under the supervision of a physician. Medications and equipment to treat severe bronchospasm must be present in the testing area. (5.1)
- Subjects with co-morbid conditions: Use with caution in patients with conditions that may increase sensitivity to the bronchoconstricting or other potential effects of ARIDOL such as: severe cough, ventilatory impairment, unstable angina, or active upper or lower respiratory tract infection that may worsen with use of a bronchial irritant. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (rate ≥1%) were headache, pharyngolaryngeal pain, throat irritation, nausea, cough, rhinorrhea, dyspnea, chest discomfort, wheezing, retching and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Methapharm, Inc. at 1-866-701-4636 or email at medinfo@methapharm.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchSee 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Bronchial Challenge Test Kit Overview
2.2 Administration Instructions
2.3 Bronchial Challenge Test Response and Patient Management
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS & PRECAUTIONS
5.1 Severe Bronchospasm
5.2 Subjects with Co-morbid Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic and Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SEVERE BRONCHOSPASM
Mannitol, the active ingredient in ARIDOL, acts as a bronchoconstrictor and may cause severe bronchospasm. Bronchial challenge testing with ARIDOL is for diagnostic purposes only. Bronchial challenge testing with ARIDOL should only be conducted by trained professionals under the supervision of a physician familiar with all aspects of the bronchial challenge test and the management of acute bronchospasm. Medications (such as short-acting inhaled beta-agonist) and equipment to treat severe bronchospasm must be present in the testing area. If severe bronchospasm occurs it should be treated immediately by administration of a short-acting inhaled beta-agonist. Because of the potential for severe bronchoconstriction, the bronchial challenge testing with ARIDOL should not be performed in any patient with clinically apparent asthma or very low baseline pulmonary function tests (e.g., FEV1 <1-1.5 liters or <70% of the predicted values) [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
ARIDOL is indicated for the assessment of bronchial hyperresponsiveness in adult and pediatric patients 6 years of age or older who do not have clinically apparent asthma.
Limitations of Use:
ARIDOL is not a standalone test or a screening test for asthma. Bronchial challenge testing with ARIDOL should be used only as part of a physician's overall assessment of asthma. -
2 DOSAGE AND ADMINISTRATION
2.1 Bronchial Challenge Test Kit Overview
ARIDOL is a bronchial challenge test kit containing the required capsules of dry powder mannitol for oral inhalation in graduated doses with the supplied single patient use inhaler necessary to perform one bronchial challenge test. Do not swallow ARIDOL capsules.
The airway response to bronchial challenge testing with ARIDOL is measured using forced expiratory volume in one second (FEV1).
Prior to bronchial challenge testing with ARIDOL, standard spirometry should be performed and the reproducibility of the resting FEV1 established.2.2 Administration Instructions
An overview of the testing procedure can be found below. The ARIDOL bronchial challenge test should only be used with the provided inhaler. All remaining unused (opened and unopened) blister packs and the inhaler should be properly discarded at the completion of the test. See the ARIDOL Bronchial Challenge Test Kit instructions for complete instructions on the dosing and spirometry procedures.
a. A nose clip may be used if preferred. If so, apply nose clip to the patient and direct the patient to breathe through the mouth
b. Insert 0 mg capsule into inhalation device. Puncture capsule by depressing buttons on side of device slowly, and ONCE ONLY (a second puncture may fragment the capsules)
c. The patient should exhale completely, before inhaling from device in a controlled deep inspiration
d. At the end of deep inspiration, start 60 second timer, subject should hold breath for 5 seconds and exhale through mouth before removal of nose clip
e. At the end of 60 seconds, measure the FEV1 in duplicate (the measurement after inhaling the 0 mg capsule is the baseline FEV1)
f. Repeat steps a-e following the mannitol capsule dose steps from Table 1 below until the patient has a positive response or 635 mg of mannitol has been administered (negative test)
Table 1: Mannitol dose steps for bronchial challenge testing with ARIDOL
Dose #
Dose mg
Cumulative Dose mg
Capsules per dose
1
0
0
1
2
5
5
1
3
10
15
1
4
20
35
1
5
40
75
1
6
80
155
2 x 40 mg
7
160
315
4 x 40 mg
8
160
475
4 x 40 mg
9
160
635
4 x 40 mg
2.3 Bronchial Challenge Test Response and Patient Management
A positive response is achieved when the patient experiences a 15% reduction in FEV1 from (0 mg) baseline (or a 10% incremental reduction in FEV1 between consecutive doses). The test result is expressed as a PD15.
Patients with either a positive response to bronchial challenge testing with ARIDOL or significant respiratory symptoms should receive a standard dose of a short-acting inhaled beta-agonist and monitored until fully recovered to within baseline.
-
3 DOSAGE FORMS AND STRENGTHS
Inhalation powder: 0 mg, 5 mg, 10 mg, 20 mg, and 40 mg of mannitol dry powder per capsule in a bronchial challenge test kit.
Each kit contains one, single patient use, dry powder inhaler device and 3 consecutively numbered foil blister packs containing a total of 19 capsules of mannitol for oral inhalation as described below:
Blister pack "1":- Marked 1 - 1 x empty clear capsule printed with two white bands
- Marked 2 - 1 x 5 mg white/clear capsule printed with 5 mg
- Marked 3 - 1 x 10 mg yellow/clear capsule printed with 10 mg
- Marked 4 - 1 x 20 mg pink/clear capsule printed with 20 mg
Blister pack "2":
- Marked 5 - 1 x 40 mg red/clear capsule printed with 40 mg
- Marked 6 - 2 x 40 mg red/clear capsules printed with 40 mg
- Marked 7 - 4 x 40 mg red/clear capsules printed with 40 mg
Blister pack "3":
- Marked 8 - 4 x 40 mg red/clear capsules printed with 40 mg
- Marked 9 - 4 x 40 mg red/clear capsules printed with 40 mg
-
4 CONTRAINDICATIONS
ARIDOL is contraindicated in:
- Patients with known hypersensitivity to mannitol or to the gelatin used to make the capsules
- Patients with conditions that may be compromised by induced bronchospasm or repeated spirometry maneuvers. Some examples include: aortic or cerebral aneurysm, uncontrolled hypertension, recent myocardial infarction or cerebral vascular accident [see Warnings and Precautions (5.2)].
-
5 WARNINGS & PRECAUTIONS
5.1 Severe Bronchospasm
Mannitol, the active ingredient in ARIDOL, acts as a bronchoconstrictor and may cause severe bronchospasm in susceptible patients. The test should only be conducted by trained professionals under the supervision of a physician familiar with all aspects of the bronchial challenge test and the management of acute bronchospasm. Patients should not be left unattended during the bronchial challenge test. Medications and equipment to treat severe bronchospasm must be present in the testing area.
If a patient has a ≥10% reduction in FEV1 (from pre-challenge FEV1) on administration of the 0 mg capsule, the ARIDOL Bronchial Challenge Test should be discontinued and the patient should be given a dose of a short-acting inhaled beta-agonist and monitored accordingly.
Patients with either a positive response to bronchial challenge testing with ARIDOL or significant respiratory symptoms should receive a short-acting inhaled beta-agonist. Patients should be monitored until fully recovered to within baseline.
5.2 Subjects with Co-morbid Conditions
Bronchial challenge testing with ARIDOL should be performed with caution in patients with conditions that may increase sensitivity to the bronchoconstricting or other potential effects of ARIDOL such as severe cough, ventilatory impairment, spirometry-induced bronchoconstriction, hemoptysis of unknown origin, pneumothorax, recent abdominal or thoracic surgery, recent intraocular surgery, unstable angina, or active upper or lower respiratory tract infection.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
• Severe Bronchospasm [see Warnings and Precautions (5.1)].6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population for the ARIDOL bronchial challenge test consisted of 1,082 subjects (577 females and 505 males) including patients with asthma, symptoms suggestive of asthma, and healthy individuals from 6 to 83 years of age who participated in the two clinical trials (Studies 1 and 2). The racial distribution of subjects was 84% Caucasian, 5% Asian, 4% Black, and 7% Other. Pediatric and adolescents patients comprised 23% of the total study population with 118 pediatric patients aged 6-11 years and 128 adolescents aged 12-17 years.
Adverse reactions were reported at the time of the testing procedure and for one day thereafter. No serious adverse reactions were reported following bronchial challenge testing with ARIDOL in either trial.
Five adult subjects (0.6%) discontinued from the studies within a day following bronchial challenge testing with ARIDOL because of cough, decreased lung function, feeling jittery, sore throat, and throat irritation. One adult subject (0.3%) discontinued following the methacholine bronchial challenge test because of dizziness. One pediatric subject (0.4%) discontinued from the studies within a day following bronchial challenge testing with ARIDOL because of retching.
Table 2 displays the combined common adverse reactions (≥1%) within a day after bronchial challenge testing with ARIDOL or methacholine in the overall population for Studies 1 and 2.
Table 2: Adverse reactions with an incidence ≥1% within a day after bronchial challenge testing (overall population, Studies 1 and 2 combined) Adverse Reactions Treatment ARIDOL
(N=1046)
n (%)Methacholine Challenge
(N=420)
n (%)Headache 59 (6) 4 (1) Pharyngolaryngeal pain 25 (2) 0 Throat irritation 19 (2) 1 (<1) Nausea 19 (2) 0 Cough 17 (2) 8 (2) Rhinorrhea 16 (2) 0 Dyspnea 15 (1) 21 (5) Chest discomfort 13 (1) 18 (4) Wheezing 8 (1) 6 (1) Retching 6 (1) 0 Dizziness 5 (1) 13 (3) The maximum reduction in FEV1 following bronchial challenge testing with ARIDOL was 46%, compared to 54% for exercise testing and 67% for the methacholine challenge. The incidences in decreases in FEV1 ≥30% and ≥60% following ARIDOL, methacholine, and exercise challenges for Studies 1 and 2 is shown in Table 3.
Table 3: Incidence of decreases in FEV1 ≥30% or ≥60% (overall population, Studies 1 and 2) Challenge No. Exposed N (%) with Fall
in FEV1 ≥30%N (%) with Fall
in FEV1 ≥60%Study 1 Exercise 435 27 (6%) 0 Methacholine 420 51 (12%) 3 (1%) ARIDOL 419 3 (1%) 0 Study 2 ARIDOL asthmatics 536 23 (4%) 0 ARIDOL Non-asthmatics 91 0 0 There were no differences in the incidence of adverse reactions based on gender or race. The clinical trials did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently compared to subjects below 65 years of age.
Pediatric Patients Aged 6 to 17 Years: Overall, the types and severities of adverse reactions in children were similar to those observed in the adult population. As in the adult population, the adverse reactions of pharyngolaryngeal pain, nausea, and headache were the more common with incidences of 4%, 3%, and 3%, respectively. There were no major differences in the types of adverse reactions observed in children 6-11 years of age compared to adolescents 12-17 years old.The decrease in FEV1 in pediatric patients and adolescents who received the ARIDOL bronchial challenge test was similar to that of the adult population with 5%, 15% and 9% of pediatric patients who had bronchial challenge testing with ARIDOL, methacholine and exercise, respectively, experiencing reduction in FEV1 ≥30%. No patient who had bronchial challenge testing with ARIDOL or exercise had a decrease in FEV1 ≥60%, whereas, one adolescent patient (aged 12 years) who received methacholine had a decrease in FEV1 ≥60%.
6.2 Post-Marketing Experience
The following adverse reactions have been identified post approval outside the U.S. of the ARIDOL Bronchial Challenge Test Kit: cough, gagging, wheeze, and decreased forced expiratory volume. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available human data regarding inhaled mannitol to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Based on animal reproduction studies, no evidence of structural alterations was observed when mannitol was orally administered to pregnant rats and mice during organogenesis at doses up to approximately 20 and 10 times, respectively, the maximum recommended daily inhalation dose (MRDID) in humans (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectivelyData
Animal Data
In animal reproduction studies, oral administration of mannitol to pregnant rats and mice during the period of organogenesis did not cause fetal structural alterations. The mannitol dose in rats and mice was approximately 20 and 10 times the maximum recommended human daily inhalation dose (MRDID) in humans, respectively, (on a mg/m2 basis at maternal doses of 1600 mg/kg/day in both
species).8.2 Lactation
Risk Summary
There are no data on the presence of mannitol in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ARIDOL bronchial challenge test and any potential adverse effects on the breastfed child from ARIDOL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ARIDOL for the assessment of bronchial hyperresponsiveness in adult and pediatric patients 6 years of age or older who do not have clinically apparent asthma have been established. The use of ARIDOL for this indication is supported by evidence from two clinical studies that included 246 pediatric patients 6 to 17 years of age [see Clinical Studies (14)].
The mean and median maximum percentage reduction in FEV1 in patients with a positive ARIDOL challenge test in pediatric patients 6 to 17 years of age (19% and 18%, respectively) showed no apparent difference compared to the adult population (19% and 18%, respectively).
The safety profile of the ARIDOL bronchial challenge test in pediatric patients 6 to 17 years of age was similar to the adult population in two clinical studies [see Adverse Reactions (6)].
Safety and effectiveness of ARIDOL have not been established in pediatric patients less than 6 years old. Bronchial challenge testing with ARIDOL should not be performed in children less than 6 years of age due to their inability to provide reliable spirometric measurements.
8.5 Geriatric Use
Clinical studies of ARIDOL did not include sufficient numbers of patients 50 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Hepatic and Renal Impairment
Formal pharmacokinetic studies with mannitol, the active ingredient, in ARIDOL, have not been conducted in patients with hepatic or renal impairment. However, an increase in systemic exposure of mannitol can be expected in patients with renal impairment based on the kidney being its primary route of elimination.
Given parenterally, mannitol is used as an osmotic diuretic in a variety of clinical situations including acute renal failure where the osmotic effects of mannitol inhibit the rate of water re-absorption and maintain the rate of urine production.
- 10 OVERDOSAGE
-
11 DESCRIPTION
D-mannitol (referred to throughout as mannitol), the active ingredient in ARIDOL is a hexahydric alcohol, that is a sugar alcohol, with the following chemical name (2R,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol and chemical structure:
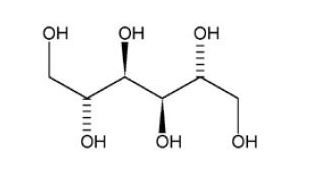
Mannitol is a white or almost white crystalline powder of free-flowing granules with an empirical formula of C6H14O6 and molecular weight of 182.2. Mannitol is freely soluble in water, and very slightly soluble in alcohol. Mannitol shows polymorphism.
The ARIDOL Bronchial Challenge Test Kit contains one single patient use dry powder inhaler and 3 consecutively numbered foil blister packs containing a total of 19 capsules of mannitol for oral inhalation. All except the 0 mg printed hard gelatin capsules contain dry powder mannitol for oral inhalation. The accompanying dry powder inhaler is a plastic device used for inhaling the capsules. All doses are to be administered using the same device supplied with each kit without washing or sterilizing the device at anytime during the test.
To use the delivery system, a mannitol capsule is placed in the well of the inhaler, and the capsule is pierced by pressing and releasing the buttons ONCE on the side of the device. The mannitol dry powder is dispersed into the air stream when the patient inhales deeply through the mouthpiece.
There are no inactive ingredients in the mannitol capsules supplied with the ARIDOL Bronchial Challenge Test Kit. The 0 mg capsule and the bodies of the 5, 10, 20 and 40 mg capsules are clear. The white caps (5 mg) contain titanium dioxide. The yellow caps (10 mg) contain titanium dioxide and yellow iron oxide. The pink caps (20 mg) and red caps (40 mg) contain titanium dioxide and red iron dioxide. The inhaler is a plastic device used for administering mannitol to the lungs. The amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow rate and inspiratory time. Under standardized in vitro testing at a fixed flow rate of 60 L/min for 2 seconds, the delivered dose from the inhaler from each of the 5, 10, 20 and 40 mg capsules is approximately 3.4, 7.7, 16.5 and 34.1 mg, respectively. Peak inspiratory flow rates (PIFR) achievable through the inhaler were evaluated in healthy and asthmatic individuals ranging from 7 to 65 years of age and with % FEV1 of predicted ranging from 67% to 123%. PIFR achieved in the study was at least 70.8 L/min in all subjects assessed. The mean PIFR was 118.2 L/min and approximately ninety percent of each population studied generated a PIFR through the device exceeding 90 L/min.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanisms through which inhaled mannitol causes bronchoconstriction are not known.
12.2 Pharmacodynamics
The response to inhaled mannitol is reported as the delivered dose of mannitol causing a 15% reduction in FEV1 and is expressed as PD15.
12.3 Pharmacokinetics
Absorption: The rate and extent of absorption of mannitol after oral inhalation was generally similar to that observed after oral administration. In a study of 18 healthy adult male subjects the absolute bioavailability of mannitol powder following oral inhalation was 59% while the relative bioavailability of inhaled mannitol in comparison to orally administered mannitol was 96%. Following oral inhalation of 635 mg, the mean mannitol peak plasma concentration (Cmax) was 13.71 mcg/mL while the mean extent of systemic exposure (AUC) was 73.15 mcg•hr/mL. The mean time to peak plasma concentration (Tmax) after oral inhalation was 1.5 hour.
Distribution: Based on intravenous administration, the volume of distribution of mannitol was 34.3 L.
Elimination: Following oral inhalation, the elimination half-life of mannitol was 4.7 hours. The mean terminal elimination half-life for mannitol in plasma remained unchanged regardless of the route of administration (oral, inhalation, and intravenous). The urinary excretion rate versus time profile for mannitol was consistent for all routes of administration. The total clearance after intravenous administration was 5.1 L/hr while the renal clearance was 4.4 L/hr. Therefore, the clearance of mannitol was predominately via the kidney. Following inhalation of 635 mg of mannitol in 18 healthy subjects, about 55% of the total dose was excreted in the urine as unchanged mannitol. Following oral or intravenous administration of a 500 mg dose, the corresponding values were 54% and 87% of the dose, respectively.
Metabolism: The extent of metabolism of mannitol appears to be small. This is evident from a urinary excretion of about 87% of unchanged drug after an intravenous dose to healthy subjects.
Specific PopulationsPatients with Hepatic and Renal Impairment: Formal pharmacokinetic studies using ARIDOL have not been conducted in patients with hepatic or renal impairment. Since the drug is eliminated primarily via the kidney, an increase in systemic exposure can be expected in renally impaired patients.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies in rats and mice, mannitol did not show evidence of carcinogenicity at oral dietary concentrations up to 5% (or 7,500 mg/kg on a mg/kg basis). These doses were approximately 55 and 30 times the MRHDID, respectively, on a mg/m2 basis.
Mannitol tested negative in the following assays: bacterial gene mutation assay, in vitro mouse lymphoma assay, in vitro chromosomal aberration assay in WI-38 human cells, in vivo chromosomal aberration assay in rat bone marrow, in vivo dominant lethal assay in rats, and in vivo mouse micronucleus assay.
The effect of inhaled mannitol on fertility has not been investigated.
-
14 CLINICAL STUDIES
The effectiveness of the ARIDOL Bronchial Challenge Test Kit in assessing bronchial hyperresponsiveness in adults and children 6 years of age and older was assessed in two clinical studies. Study 1 was an operator-blinded, open-label crossover trial that assessed the sensitivity and specificity of bronchial challenge testing with ARIDOL compared with a methacholine bronchial challenge test in detecting bronchial hyperresponsiveness in subjects with symptoms suggestive of asthma but without a definite diagnosis of asthma. During the course of the study subjects underwent three types of bronchial challenge tests utilizing exercise, ARIDOL, and methacholine. A positive exercise test was defined as a decrease in FEV1 ≥10%, a positive bronchial challenge test with ARIDOL was defined by either a decrease in FEV1 by ≥15% from baseline or a between-dose reduction in FEV1 ≥10%, and a positive methacholine response was defined as a decrease in FEV1 ≥20% after breathing methacholine at a concentration less than or equal to 16 mg/mL. The sensitivity and specificity of bronchial challenge testing with ARIDOL and methacholine were then assessed relative to exercise testing which served as a common comparator. The sensitivity and specificity of ARIDOL and methacholine challenges were also assessed using a blinded study physician's diagnosis of asthma at the end of the study. Five-hundred nine subjects aged 6 to 50 years were screened for enrolment with 419 and 420 subjects receiving at least one dose of mannitol, the active ingredient in ARIDOL, or methacholine, respectively. The maximum cumulative dose of mannitol was 635 mg. Bronchial challenge testing with ARIDOL and methacholine demonstrated similar sensitivity and specificity in predicting bronchial hyperresponsiveness defined by a positive exercise challenge (Table 4).
Table 4: Comparisons of the sensitivity and specificity (calculated relative to exercise challenge) for the ARIDOL test and methacholine in Study 1 Population Treatment Sensitivity %
(95% CI)Specificity %
(95% CI)Overall Population (n=419) ARIDOL 58 (50, 65) 63 (57, 69) Methacholine 53 (46, 51) 68 (62, 73) Difference 5 (-4, 13) -5 (-12, 3) Age 6-11 years old (n=36) ARIDOL 67 (47, 87) 47 (21, 72) Methacholine 71 (52, 91) 33 (9, 57) Difference -5 (-29, 20) 17 (-29, 62) Age 12-17 years old (n=70) ARIDOL 55 (37, 72) 62 (46, 77) Methacholine 65 (48, 81) 64 (49, 79) Difference -10 (32, 13) -3 (-24, 19) Bronchial challenge testing with ARIDOL and methacholine also demonstrated similar sensitivity and specificity when calculated relative to a blinded study physician's diagnosis of asthma in subjects at the end of the study.
The sensitivity and specificity of bronchial challenge testing with ARIDOL in children and adolescents 6 to 17 years of age in Study 1 was similar to that in the overall population (Table 4).
Study 2 was a crossover study comparing bronchial challenge testing with ARIDOL to hypertonic (4.5%) saline in identifying bronchial hyperresponsiveness in subjects 6 to 83 years of age with (n=551) and without (n=95) asthma. In this study the efficacy endpoint of interest was an estimation of the sensitivity and specificity of bronchial challenge testing with ARIDOL with respect to a physician's clinical diagnosis of asthma. Following completion of the bronchial challenge tests with ARIDOL and hypertonic saline, a respiratory physician assessed the data and categorized the subjects as having or not having asthma. The sensitivity of the ARIDOL bronchial challenge test in subjects with a physician diagnosis of asthma was 58% [(54%, 62%, 95th CI)] compared to a sensitivity of the physician diagnosis in the same population of 97% [(95%, 98%, 95th CI)]. The specificity of the ARIDOL bronchial challenge test in subjects without asthma was 95% [(90%, 99%, 95th CI)] compared to the specificity of the physician diagnosis of 98% [(95%, 100%, 95th CI)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ARIDOL is a bronchial challenge test kit. Each kit contains one single patient use, dry powder inhaler device and 3 consecutively numbered foil blister packs containing a total of 19 capsules of dry powder mannitol for oral inhalation as described below:
Blister pack "1":- Marked 1 - 1 x empty clear capsule printed with two white bands
- Marked 2 - 1 x 5 mg white/clear capsule printed with 5 mg
- Marked 3 - 1 x 10 mg yellow/clear capsule printed with 10 mg
- Marked 4 - 1 x 20 mg pink/clear capsule printed with 20 mg
Blister pack "2":
- Marked 5 - 1 x 40 mg red/clear capsule printed with 40 mg
- Marked 6 - 2 x 40 mg red/clear capsules printed with 40 mg
- Marked 7 - 4 x 40 mg red/clear capsules printed with 40 mg
Blister pack "3":
- Marked 8 - 4 x 40 mg red/clear capsules printed with 40 mg
- Marked 9 - 4 x 40 mg red/clear capsules printed with 40 mg
NDC-67850-552-01
Storage
Store below 77°F (25°C) with excursions permitted between 59°F-86°F (15°C-30°C). [See USP Controlled Room Temperature]. Do not freeze. Do not refrigerate.
-
17 PATIENT COUNSELING INFORMATION
Severe Bronchospasm
Prior to administration patients should be informed of the potential for bronchial challenge testing with ARIDOL to cause severe bronchospasm and of the potential symptoms they may experience [see Warnings and Precautions (5.1)].
Patients with Certain Co-morbid Conditions
Bronchial challenge testing with ARIDOL should be performed with caution in patients having severe cough, ventilatory impairment, spirometry-induced bronchoconstriction, hemoptysis of unknown origin, pneumothorax, recent abdominal or thoracic surgery, recent intraocular surgery, unstable angina, or active upper or lower respiratory tract infection or other conditions that may worsen with the use of a bronchial irritant [see Warnings and Precautions (5.2)].
Manufactured by:
Pharmaxis Ltd
20 Rodborough Rd
Frenchs Forest NSW 2086
AUSTRALIA
Manufactured for:
Methapharm, Inc.
11772 West Sample Road, Suite 101 Coral Springs,
FL, 33065 USA
1-833-887-7686 www.aridolchallenge.com
ussales@methapharm.com
ARIDOL® is a registered trademark of Pharmaxis Ltd
-
Aridol kit carton
PRINCIPAL DISPLAY PANEL
NDC 67850-552-01
Rx Only
Caution: Federal Law Requires Test To Be Administered By a Trained Healthcare Professional Only
Do Not Swallow Aridol Capsulesaridol™
(mannitol inhalation powder)
Bronchial Challenge Test KitFOR ORAL INHALATION ONLY
One complete diagnostic kit to measure bronchial hyperresponsiveness
Contents:
3 Blister Cards:
0 mg - 1 capsule
5 mg - 1 capsule
10 mg - 1 capsule
20 mg - 1 capsule
40 mg - 15 capsules
1 Aridol device: For use with enclosed capsules onlySee package insert for dosage information.
-
INGREDIENTS AND APPEARANCE
ARIDOL BRONCHIAL CHALLENGE TEST KIT
mannitol kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67850-552 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67850-552-01 1 in 1 KIT; Type 0: Not a Combination Product 10/05/2010 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 KIT 1 Part 2 1 KIT 1 Part 3 1 KIT 1 Part 4 1 CAPSULE 1 Part 1 of 4 ARIDOL
mannitol powderProduct Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 5 mg Product Characteristics Color white (white/clear) Score no score Shape capsule Size 15mm Flavor Imprint Code 5;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022368 10/05/2010 Part 2 of 4 ARIDOL
mannitol powderProduct Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 10 mg Product Characteristics Color yellow (yellow/clear) Score no score Shape capsule Size 15mm Flavor Imprint Code 10;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022368 10/05/2010 Part 3 of 4 ARIDOL
mannitol powderProduct Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 20 mg Product Characteristics Color pink (pink/clear) Score no score Shape capsule Size 15mm Flavor Imprint Code 20;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022368 10/05/2010 Part 4 of 4 ARIDOL
mannitol powderProduct Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 40 mg Product Characteristics Color red (red/clear) Score no score Shape capsule Size 15mm Flavor Imprint Code 40;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022368 10/05/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022368 10/05/2010 Labeler - Methapharm, Inc. (066672887) Establishment Name Address ID/FEI Business Operations Pharmaxis Ltd 744446795 manufacture(67850-552)