Label: OMECLAMOX-PAK- omeprazole, clarithromycin, amoxicillin kit
- NDC Code(s): 66220-422-01, 66220-422-02
- Packager: Cumberland Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMECLAMOX®-PAK safely and effectively. See full prescribing information for OMECLAMOX-PAK.
OMECLAMOX-PAK (omeprazole delayed-release capsules; clarithromycin tablets, amoxicillin capsules) co-packaged for oral use
Initial U.S. Approval: 2011RECENT MAJOR CHANGES
Contraindications (4.2) 08/2023 INDICATIONS AND USAGE
OMECLAMOX-PAK, a co-packaged product containing omeprazole, a proton pump inhibitor, clarithromycin, a macrolide antimicrobial, and amoxicillin, a penicillin class antibacterial, is indicated for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or up to one-year history) to eradicate H. pylori. (1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of OMECLAMOX-PAK and other antibacterial drugs, OMECLAMOX-PAK should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.2)
DOSAGE AND ADMINISTRATION
- Adult regimen: omeprazole 20 mg plus clarithromycin 500 mg plus amoxicillin 1000 mg, each given twice daily for 10 days in the morning and evening before eating a meal. (2)
- Advise patients to swallow all tablets and capsules whole. (2)
- In patients with an ulcer present at initiation of therapy, an additional 18 days of omeprazole 20 mg once daily is recommended. (2)
DOSAGE FORMS AND STRENGTHS
Pack of 10 daily administration cards for morning and evening dosing, each containing (3):
- Two omeprazole delayed-release capsules, USP, 20 mg.
- Two clarithromycin tablets, USP, 500 mg.
- Four amoxicillin capsules, USP, 500 mg.
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Embryo-Fetal Toxicity: Based on animal findings for omeprazole and clarithromycin, OMECLAMOX-PAK may cause fetal harm. OMECLAMOX-PAK is not recommended for use in pregnant women except in clinical circumstances when there is no appropriate alternative therapy. (5.1)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.2)
- Colchicine interaction: Concomitant use of clarithromycin and colchicine has resulted in deaths, especially in the elderly with renal insufficiency. Monitor patients for clinical symptoms of colchicine toxicity. (5.3, 7.1)
- Myasthenia gravis: Exacerbation of symptoms and new onset of symptoms reported with clarithromycin. Monitor patients for symptoms. (5.4)
- Clostridioides difficile-associated diarrhea: Reported with use of clarithromycin and amoxicillin; evaluate if diarrhea occurs. (5.5)
- Risk of gastric malignancy: Symptomatic response does not preclude concomitant underlying malignancy. (5.6)
- Acute Tubulointerstitial Nephritis: has been observed in patients taking proton pump inhibitors (PPIs), including omeprazole. Discontinue OMECLAMOX-PAK and evaluate patients (5.7)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue OMECLAMOX- PAK and evaluate (5.8)
ADVERSE REACTIONS
Most frequent adverse reactions (> 7%) with triple therapy were diarrhea, taste perversion, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Cumberland Pharmaceuticals Inc. at 1-877-484-2700 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Antiarrhythmics: Risk of torsades de pointes and other arrhythmias with concurrent use of clarithromycin and quinidine, disopyramide, and digoxin. Monitor ECGs and serum digoxin concentrations. (7.4)
- Oral anticoagulants: Concomitant administration of omeprazole or clarithromycin may potentiate the anticoagulant effects of warfarin and other oral anticoagulants. Monitor prothrombin times and INR. (7.5)
- Atazanavir and nelfinavir: Omeprazole reduces plasma concentrations of atazanavir and nelfinavir. Concomitant use is not recommended. (7.6)
- Saquinavir: Omeprazole increases plasma concentrations of saquinavir. Monitor for toxicity and consider dose reduction of saquinavir. (7.6)
- Cilostazol: Omeprazole increases systemic exposure of cilostazol and one of its active metabolites. Consider dose reduction of cilostazol. (7.7)
- Tacrolimus: Omeprazole may increase serum concentrations of tacrolimus. Frequently monitor whole blood trough concentrations of tacrolimus. (7.8)
- Theophylline: Clarithromycin may increase serum concentrations of theophylline. Monitor serum theophylline concentrations. (7.9)
- Carbamazepine: Clarithromycin may increase plasma concentrations of carbamazepine. Monitor blood concentrations of carbamazepine. (7.10)
- Sildenafil: Clarithromycin may increase systemic exposure of sildenafil. Consider dose reduction of sildenafil. (7.11)
- HMG-CoA reductase inhibitors (statins): Clarithromycin may alter the effect of HMG-CoA reductase inhibitors (statins). (7.12)
- Drugs metabolized by cytochrome P450 (e.g., diazepam, warfarin, phenytoin, cyclosporine, disulfiram, benzodiazepines): Omeprazole can prolong their elimination. Monitor and determine need for dose adjustments. (7, 7.13)
- Probenecid: Probenecid may increase blood concentrations of the amoxicillin. (7.14)
- Omeprazole may interfere with drugs for which gastric pH affects bioavailability (e.g., ketoconazole, iron salts, ampicillin esters, digoxin, and mycophenolate mofetil). (7.15)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
1.2 Usage
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Serious Drug Interactions
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 Severe Cutaneous Adverse Reactions
5.3 Colchicine Toxicity with Clarithromycin
5.4 Myasthenia Gravis
5.5 Clostridioides difficile-associated diarrhea
5.6 Concomitant Gastric Malignancy
5.7 AcuteTubulointerstitial Nephritis
5.8 Cutaneous and Systemic Lupus
5.9 Development of Bacterial Superinfections
5.10 Mononucleosis and Ampicillin
5.11 Development of Drug Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Adverse Reactions from Labeling for the Individual Components of OMECLAMOX-PAK
6.3 Post-Marketing Experience with the Individual Components of OMECLAMOX-PAK
7 DRUG INTERACTIONS
7.1 Colchicine
7.2 Ergotamine/Dihydroergotamine
7.3 Pimozide
7.4 Antiarrhythmics
7.5 Anticoagulants
7.6 Antiretroviral Drugs
7.7 Cilostazol
7.8 Tacrolimus
7.9 Theophylline
7.10 Carbamazepine
7.11 Sildenafil
7.12 HMG-CoA Reductase Inhibitors (Statins)
7.13 Triazolobenzodiazepines (e.g., triazolam and alprazolam) and related Benzodiazepines (e.g., midazolam)
7.14 Probenecid
7.15 Drugs for which Gastric pH can affect Bioavailability
7.16 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Asian Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 H. pylori associated Duodenal Ulcer Disease
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
OMECLAMOX-PAK (Omeprazole delayed-release capsules, clarithromycin tablets, and amoxicillin capsules taken together) are indicated for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or one-year history) to eradicate H. pylori in adults. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14.1)].
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of OMECLAMOX-PAK and other antibacterial drugs OMECLAMOX-PAK should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
The recommended adult oral dose and regimen is omeprazole delayed-release capsules 20 mg plus clarithromycin 500 mg plus amoxicillin 1000 mg, each given twice daily, for 10 days, in the morning and evening before eating a meal. Inform patients that omeprazole, clarithromycin, and amoxicillin should not be crushed or chewed, and should be swallowed whole.
In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief.
-
3 DOSAGE FORMS AND STRENGTHS
OMECLAMOX-PAK is supplied in a carton containing ten individual daily administration cards. Each card contains:
- Omeprazole Delayed-Release Capsules, USP, 20 mg
Two opaque, hard gelatin lavender and grey capsules, with ‘R 158’ and ‘OMEPRAZOLE 20 mg’ imprinted on the capsules in black ink, containing off-white to pale-yellow, elliptical spherical pellets.
- Clarithromycin Tablets, USP, 500 mg
Two white, biconvex beveled-edge capsule-shaped coated tablets debossed with ‘54 312’ on one side and plain on the other side.
- Amoxicillin Capsules, USP, 500 mg
Four opaque hard gelatin yellow capsules, marked ‘GG849’.
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
OMECLAMOX-PAK is contraindicated in the following patients:
- Known history of hypersensitivity to omeprazole or benzimidazoles, any macrolide antibacterial drug, or any penicillin. Hypersensitivity reactions to omeprazole may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute tubulointerstitial nephritis, and urticaria. Hypersensitivity reactions to clarithromycin may include anaphylaxis, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Hypersensitivity reactions to amoxicillin may include serum sickness like reactions, erythematous maculopapular rashes, erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis and urticaria [see Warnings and Precautions (5.7), Adverse Reactions (6.3)].
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients on oral penicillins. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. Before initiating therapy with amoxicillin, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins or other allergens.
4.2 Serious Drug Interactions
- OMECLAMOX-PAK is contraindicated in patients taking ergotamine or dihydroergotamine and pimozide. Cardiac arrhythmias, some fatal, have been reported with the use of clarithromycin and/or erythromycin and pimozide. Arrhythmias have included QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes, and are most likely due to inhibition of metabolism of these drugs by clarithromycin and/or erythromycin [see Drug Interactions (7.2, 7.3)].
- Coadministration of OMECLAMOX-PAK with lurasidone is contraindicated since it may result in an increase in lurasidone expose and the potential for serious adverse reactions [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Clarithromycin, a component of OMECLAMOX-PAK, has demonstrated adverse effects on pregnancy outcomes and/or embryo-fetal development in monkeys, rats, mice, and rabbits at doses that produced plasma concentrations 2 to 17 times the serum concentrations achieved in humans at the maximum recommended human dose.
Based on findings in animal studies, OMECLAMOX-PAK is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate. If OMECLAMOX-PAK is used during pregnancy, or if pregnancy occurs while the patient is taking this drug, the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
5.2 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the components of OMECLAMOX-PAK: omeprazole, clarithromycin, and amoxicillin [see Adverse Reactions (6.3)].
Discontinue OMECLAMOX-PAK at the first signs or symptoms of SCAR or other signs of hypersensitivity and consider further evaluation.
5.3 Colchicine Toxicity with Clarithromycin
There have been postmarketing reports of colchicine toxicity, some fatal, with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Monitor patients for clinical symptoms of colchicine toxicity [see Drug Interactions (7.1)].
5.4 Myasthenia Gravis
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome have been reported in patients receiving clarithromycin therapy. Monitor patients for symptoms.
5.5 Clostridioides difficile-associated diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of clarithromycin and amoxicillin, and may range in severity from mild diarrhea to fatal colitis [see Adverse Reactions (6.3)]. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.6 Concomitant Gastric Malignancy
Symptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.
5.7 AcuteTubulointerstitial Nephritis
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs including omeprazole, a component of OMECLAMOX-PAK. TIN may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions, to non-specific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia). Discontinue OMECLAMOX-PAK and evaluate patients with suspected acute TIN [see Contraindications (4.1)].
5.8 Cutaneous and Systemic Lupus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including omeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving OMECLAMOX-PAK, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serological testing (e.g. ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.
5.9 Development of Bacterial Superinfections
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy with OMECLAMOX-PAK due to the clarithromycin and amoxicillin components. If superinfections occur, OMECLAMOX-PAK should be discontinued and appropriate therapy instituted.
5.10 Mononucleosis and Ampicillin
A high percentage of patients with mononucleosis who receive ampicillin develop an erythematosus skin rash. Thus, administration of ampicillin-class antibiotics is not recommended in patients with mononucleosis.
5.11 Development of Drug Resistant Bacteria
Prescribing OMECLAMOX-PAK (clarithromycin or amoxicillin component) in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity [see Contraindications (4.1)]
- Myasthenia Gravis [see Warnings and Precautions (5.4)]
- Clostridioides difficile-associated diarrhea [see Warnings and Precautions (5.5)]
- Acute Tubulointerstitial Nephritis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials using triple therapy with omeprazole, clarithromycin, and amoxicillin, no adverse reactions unique to triple therapy were observed. Adverse reactions observed were limited to those previously reported with omeprazole, clarithromycin, or amoxicillin alone. The most frequent adverse reactions observed in clinical trials using combination therapy with omeprazole, clarithromycin, and amoxicillin (n = 274) were diarrhea (14%), taste perversion (10%), and headache (7%). None of these occurred at a higher frequency than that reported by patients taking antimicrobial agents alone.
6.2 Adverse Reactions from Labeling for the Individual Components of OMECLAMOX-PAK
The safety data below reflect exposure to omeprazole delayed-release capsules and clarithromycin worldwide in clinical trials for various indications using doses and durations of therapy that may differ from how they are used as a component of OMECLAMOX-PAK. For complete information on these reactions, see the full prescribing information for omeprazole delayed-release capsules and clarithromycin.
Omeprazole:
The most common adverse reactions reported (i.e., with an incidence rate ≥ 2%) in 3096 patients from omeprazole delayed-release capsules-treated patients enrolled in clinical trials included headache (6.9%), abdominal pain (5.2%), nausea (4.0%), diarrhea (3.7%), vomiting (3.2%), and flatulence (2.7%).
Additional adverse reactions that were reported with an incidence rate ≥ 1% included acid regurgitation (1.9%), upper respiratory infection (1.9%), constipation (1.5%), dizziness (1.5%), rash (1.5%), asthenia (1.3%), back pain (1.1%), and cough (1.1%).
The clinical trial safety profile in patients greater than 65 years of age was similar to that in patients 65 years of age or less.
Clarithromycin:
The most frequently reported events in adults were diarrhea (3%), nausea (3%), abnormal taste (3%), dyspepsia (2%), abdominal pain/discomfort (2%), and headache (2%). Most of these events were described as mild or moderate in severity. Of the reported adverse events, only 1% were described as severe. Fewer than 3% of adult patients without mycobacterial infections discontinued therapy because of drug-related side effects.
6.3 Post-Marketing Experience with the Individual Components of OMECLAMOX-PAK
Because these reactions are voluntarily reported from a population of uncertain size, it is not always possible to reliably estimate their actual frequency or establish a causal relationship to drug exposure.
Omeprazole:
Body As a Whole: Hypersensitivity reactions including anaphylaxis, anaphylactic shock, angioedema, bronchospasm, interstitial nephritis, urticaria, (see also Skin below); fever; pain; fatigue; malaise.
Cardiovascular: Chest pain or angina, tachycardia, bradycardia, palpitations, elevated blood pressure, peripheral edema.
Endocrine: Gynecomastia.
Gastrointestinal: Pancreatitis (some fatal), anorexia, irritable colon, fecal discoloration, esophageal candidiasis, mucosal atrophy of the tongue, stomatitis, abdominal swelling, dry mouth. During treatment with omeprazole, gastric fundic gland polyps have been noted rarely. These polyps are benign and appear to be reversible when treatment is discontinued. Gastroduodenal carcinoids have been reported in patients with Zollinger-Ellison syndrome on long-term treatment with omeprazole. This finding is believed to be a manifestation of the underlying condition, which is known to be associated with such tumors.
Hepatic: Liver disease including hepatic failure (some fatal), liver necrosis (some fatal), hepatic encephalopathy hepatocellular disease, cholestatic disease, mixed hepatitis, jaundice, and elevations of liver function tests [ALT, AST, GGT, alkaline phosphatase, and bilirubin].
Metabolic/Nutritional: Hypoglycemia, hypomagnesemia with or without hypocalcemia and/or hypokalemia, hyponatremia, weight gain.
Musculoskeletal: Muscle weakness, myalgia, muscle cramps, joint pain, leg pain.
Nervous System/Psychiatric: Psychiatric and sleep disturbances including depression, agitation, aggression, hallucinations, confusion, insomnia, nervousness, apathy, somnolence, anxiety, and dream abnormalities; tremors, paresthesia; vertigo.
Respiratory: Epistaxis, pharyngeal pain.
Skin: SCAR, including TEN (some fatal), SJS, DRESS, and AGEP; erythema multiforme; photosensitivity; urticaria; rash; skin inflammation; pruritus; petechiae; purpura; alopecia; dry skin; hyperhidrosis.
Special Senses: Tinnitus, taste perversion.
Ocular: Optic atrophy, anterior ischemic optic neuropathy, optic neuritis, dry eye syndrome, ocular irritation, blurred vision, double vision.
Urogenital: Interstitial nephritis, hematuria, proteinuria, elevated serum creatinine, microscopic pyuria, urinary tract infection, glycosuria, urinary frequency, testicular pain, erectile dysfunction.
Hematologic: Agranulocytosis (some fatal), hemolytic anemia, pancytopenia, neutropenia, anemia, thrombocytopenia, leukopenia, leukocytosis.
Clarithromycin:
Hypersensitivity Reactions: Allergic reactions ranging from urticaria and mild skin eruptions to rare cases of anaphylaxis have occurred.
Gastrointestinal: Glossitis, stomatitis, oral moniliasis, anorexia, vomiting, pancreatitis, tongue discoloration.
Hematologic: Thrombocytopenia, leukopenia, neutropenia.
Other: There have been reports of tooth discoloration in patients treated with clarithromycin. Tooth discoloration is usually reversible with professional dental cleaning.
Nervous System/Psychiatric: There have been isolated reports of hearing loss, which is usually reversible, occurring chiefly in elderly women. Reports of alterations of the sense of smell, usually in conjunction with taste perversion or taste loss have also been reported.
Transient CNS events including anxiety, behavioral changes, confusional states, convulsions, depersonalization, disorientation, hallucinations, insomnia, manic behavior, nightmares, psychosis, tinnitus, tremor, dizziness and vertigo have been reported during postmarketing surveillance. Events usually resolve with discontinuation of the drug.
Hepatic: Hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, has been infrequently reported with clarithromycin. This hepatic dysfunction may be severe and is usually reversible. In very rare instances, hepatic failure with fatal outcome has been reported and generally has been associated with serious underlying diseases and/or concomitant medications.
Metabolic: There have been rare reports of hypoglycemia, some of which have occurred in patients taking oral hypoglycemic agents or insulin.
Cardiac: As with other macrolides, clarithromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes.
Renal: There have been reports of interstitial nephritis coincident with clarithromycin use.
Skin: SCAR, including TEN, SJS, DRESS, and AGEP.
Amoxicillin:
Gastrointestinal: Nausea, vomiting, diarrhea, anorexia, gastritis, black hairy tongue, glossitis, stomatitis, and hemorrhagic/C.difficile-associated colitis. [see Warnings and Precautions (5.5)].
Hypersensitivity Reactions: Serum sickness like reactions, erythematous maculopapular rashes, erythema multiforme, exfoliative dermatitis, hypersensitivity vasculitis and urticaria have been reported. Reactions are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillins and in those with a history of allergy, asthma, hay fever, or urticaria.
Hepatic: A moderate rise in AST (SGOT) and/or ALT (SGPT) has been noted, but the significance of this finding is unknown. Hepatic dysfunction including cholestatic jaundice, hepatic cholestasis and acute cytolytic hepatitis have been reported.
Renal: Crystalluria has also been reported [see Overdosage (10)].
Hemic and Lymphatic Systems: Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Nervous System/Psychiatric: Reversible hyperactivity, agitation, anxiety, insomnia, confusion, aseptic meningitis, behavioral changes, and/or dizziness have been reported rarely.
Miscellaneous: Tooth discoloration (brown, yellow, or gray staining) has been rarely reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
Skin: SCAR, including TEN, SJS, DRESS, and AGEP.
Changes in Laboratory Values: Changes in laboratory values with possible clinical significance were as follows: Hepatic – elevated SGPT (ALT) less than 1%, SGOT (AST) less than 1%, GGT less than 1%, alkaline phosphatase less than 1%, LDH less than 1%, total bilirubin less than 1%; Hematologic – decreased WBC less than 1%, elevated prothrombin time 1%; Renal – elevated BUN 4%, elevated serum creatinine less than 1%. GGT, alkaline phosphatase, and prothrombin time data are from adult studies only.
-
7 DRUG INTERACTIONS
Effect of Omeprazole
Omeprazole is a substrate and an inhibitor of CYP2C19 in vivo, a substrate of CYP3A4 in vivo, and an inhibitor of CYP2C19 in vitro. Therefore, omeprazole may affect the metabolism and plasma concentrations of drugs that are metabolized by these CYP enzymes. Although in healthy subjects no interaction with theophylline or propranolol was reported, there have been reports of an interaction with other drugs metabolized via the CYP enzyme system (e.g., cyclosporine, disulfiram, benzodiazepines). Carefully monitor patients taking these drugs to determine if dosage adjustments of these drugs are necessary when taken concomitantly with omeprazole.
Effect of Clarithromycin
Clarithromycin is a substrate and inhibitor of CYP3A enzymes. Coadministration of clarithromycin with drugs metabolized by CYP3A may be associated with elevations in drug concentrations that could increase the therapeutic and adverse effects of the concomitant drug. There have been reports of CYP3A-based interactions of erythromycin and/or clarithromycin with cyclosporine, tacrolimus, alfentanil, rifabutin, methylprednisolone, cilostazol, bromocriptine and lurasidone. OMECLAMOX_PAK is contraindicated in patients receiving lurasidone [see Contraindications (4.2)]. In addition, there have been reports of interactions of erythromycin or clarithromycin with drugs not thought to be metabolized by CYP3A, including: hexobarbital, phenytoin, and valproate.
7.1 Colchicine
Concurrent use of colchicine and OMECLAMOX-PAK may increase plasma colchicine concentrations. Colchicine is a substrate for both CYP3A and the efflux transporter, P-glycoprotein (Pgp). The clarithromycin component of OMECLAMOX-PAK is known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered together, inhibition of Pgp and/or CYP3A by clarithromycin may lead to increased plasma exposure to colchicine. Monitor patients for clinical symptoms of colchicine toxicity [see Warnings and Precautions (5.3)].
7.2 Ergotamine/Dihydroergotamine
Ergotamine/dihydroergotamine plasma concentrations may increase when administered concomitantly with OMECLAMOX-PAK. Post-marketing reports indicate that coadministration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. Concomitant administration of clarithromycin with ergotamine or dihydroergotamine is contraindicated [see Contraindications (4.2)].
7.3 Pimozide
The coadministration of pimozide and OMECLAMOX-PAK may increase the pimozide plasma concentrations due to an interaction with the clarithromycin component of OMECLAMOX-PAK. Post-marketing reports indicate that coadministration of clarithromycin with pimozide has been associated with cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes). Two sudden deaths have been reported when clarithromycin was added to ongoing pimozide therapy. Pimozide is metabolized partly by CYP3A4. When clarithromycin and pimozide are administered together, inhibition of CYP3A4 by clarithromycin may lead to increased plasma exposure to pimozide. OMECLAMOX-PAK is contraindicated in patients receiving pimozide [see Contraindications (4.2)].
7.4 Antiarrhythmics
Concurrent use of antiarrhythmic drugs and OMECLAMOX-PAK may potentiate the antiarrhythmic effects due to an interaction with the clarithromycin component of OMECLAMOX-PAK. There have been post-marketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have also been reported in post-marketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Monitor electrocardiograms for QTc prolongation during coadministration of OMECLAMOX-PAK with antiarrhythmic drugs. Serum concentrations of antiarrhythmics, including digoxin, should also be monitored.
7.5 Anticoagulants
The simultaneous administration of anticoagulants and OMECLAMOX-PAK may alter the anticoagulant effects of warfarin and other oral anticoagulants due to an interaction with the omeprazole and clarithromycin components of OMECLAMOX-PAK. Monitor prothrombin time and INR in patients receiving OMECLAMOX-PAK and oral anticoagulants simultaneously.
There have been reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Spontaneous reports in the post-marketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants.
7.6 Antiretroviral Drugs
Concurrent use of antiretroviral agents and OMECLAMOX-PAK may alter the antiretroviral effects due to interactions with the omeprazole or clarithromycin components of OMECLAMOX-PAK. Omeprazole has been reported to interact with some antiretroviral drugs such as atazanavir, nelfinavir, and saquinavir.
Concomitant use of atazanavir or nelfinavir with omeprazole is not recommended unless the benefits of taking atazanavir or nelfinavir with OMECLAMOX-PAK outweigh the risks. Coadministration of atazanavir or nelfinavir with proton pump inhibitors is expected to substantially decrease atazanavir or nelfinavir plasma concentrations and thereby reduce the therapeutic effect of either of these drugs [see Clinical Pharmacology (12.3)].
Coadministration of saquinavir with omeprazole may increase the serum concentrations of saquinavir. Dose reduction of saquinavir should be considered when coadministered with OMECLAMOX-PAK [see Clinical Pharmacology (12.3)].
7.7 Cilostazol
Concomitant administration of OMECLAMOX-PAK and cilostazol may increase systemic exposure of cilostazol due to an interaction with the omeprazole component of OMECLAMOX-PAK. Therefore, a dose reduction of cilostazol by 50% should be considered when concomitantly administered with OMECLAMOX-PAK [see Clinical Pharmacology (12.3)].
7.8 Tacrolimus
Concomitant administration of OMECLAMOX-PAK and tacrolimus may increase the serum concentrations of tacrolimus due to an interaction with the omeprazole component of OMECLAMOX-PAK. Frequent monitoring of whole blood trough concentrations of tacrolimus is recommended when concomitantly administered with OMECLAMOX-PAK.
7.9 Theophylline
OMECLAMOX-PAK use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations due to an interaction with the clarithromycin component of OMECLAMOX-PAK. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range [see Clinical Pharmacology (12.3)].
7.10 Carbamazepine
The simultaneous administration of carbamazepine and OMECLAMOX-PAK may alter the effect of carbamazepine due to an interaction with the clarithromycin component of OMECLAMOX-PAK. Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine should be considered when administered concomitantly with OMECLAMOX-PAK.
7.11 Sildenafil
The systemic exposure of sildenafil may increase when it is administered concomitantly with OMECLAMOX-PAK due to an interaction with the clarithromycin component of OMECLAMOX-PAK; consider a reduction in sildenafil dosage (see sildenafil full prescribing information).
7.12 HMG-CoA Reductase Inhibitors (Statins)
Concurrent use of HMG-CoA reductase inhibitors (statins) and OMECLAMOX-PAK may alter the effect of HMG-CoA due to an interaction with the clarithromycin component of OMECLAMOX-PAK. As with other macrolides, clarithromycin has been reported to increase concentrations of statins (e.g., lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
7.13 Triazolobenzodiazepines (e.g., triazolam and alprazolam) and related Benzodiazepines (e.g., midazolam)
The effect of triazolobenzodiazepines/related benzodiazepines may be altered when administered concomitantly with OMECLAMOX-PAK due to an interaction with the clarithromycin component. There have been postmarketing reports of drug interactions and CNS effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam.
7.14 Probenecid
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of OMECLAMOX-PAK and probenecid may result in increased and prolonged blood concentrations of the amoxicillin component of OMECLAMOX-PAK.
7.15 Drugs for which Gastric pH can affect Bioavailability
Due to its effects on gastric acid secretion, omeprazole can reduce the absorption of drugs where gastric pH is an important determinant of their bioavailability. As with other drugs that decrease the intragastric acidity, the absorption of drugs such as ketoconazole, atazanavir, iron salts, erlotinib, and mycophenolate mofetil can decrease, while the absorption of drugs such as digoxin can increase during treatment with omeprazole.
Concomitant treatment with omeprazole (20 mg daily) and digoxin in healthy subjects increased the bioavailability of digoxin by 10% (30% in two subjects). Coadministration of digoxin with omeprazole is expected to increase the systemic exposure of digoxin. Therefore, patients may need to be monitored when digoxin is taken concomitantly with omeprazole.
Coadministration of omeprazole in healthy subjects and in transplant patients receiving mycophenolate mofetil has been reported to reduce the exposure to mycophenolic acid (MPA), the active moiety, possibly due to a decrease in MPA solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving proton pump inhibitors (PPIs) and mycophenolate mofetil. Use omeprazole with caution in transplant patients receiving mycophenolate mofetil [see Clinical Pharmacology (12.3)].
7.16 Drug-Laboratory Test Interactions
High urine concentrations of ampicillin may result in false-positive reactions when testing for the presence of glucose in urine using glucose tests based on the Benedict's copper reduction reaction that determines the amount of reducing substances like glucose in urine. Since this effect may also occur with amoxicillin, it is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
Following administration of ampicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estradiol, estriol-glucuronide, conjugated estrone, and estradiol has been noted. This effect may also occur with amoxicillin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies for omeprazole and clarithromycin (components of OMECLAMOX-PAK), use of OMECLAMOX-PAK during pregnancy may cause fetal harm. OMECLAMOX-PAK is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate. If OMECLAMOX-PAK is used during pregnancy, or if pregnancy occurs while taking OMECLAMOX-PAK, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.1)]. There are no adequate and well controlled studies of omeprazole, clarithromycin, or amoxicillin (used separately or together) in pregnant women. Clarithromycin demonstrated adverse developmental effects in four animal species at clinically relevant doses. Omeprazole increased embryo-fetal loss in rabbits, but animal studies and multiple human studies do not show an increased risk for major malformations. There was no evidence of harm to the fetus in mice and rats due to amoxicillin.
Omeprazole
There are no adequate and well-controlled studies with omeprazole in pregnant women. Available epidemiologic data fail to demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use. Reproduction studies in rats and rabbits resulted in dose-dependent embryo-lethality at omeprazole doses that were approximately 3.4 to 34 times an oral human dose of 40 mg.
No fetal malformations were observed in animal reproduction studies with administration of oral esomeprazole (an enantiomer of omeprazole) magnesium in rats and rabbits during organogenesis with doses about 68 times and 42 times, respectively, an oral human dose of 40 mg esomeprazole or 40 mg omeprazole. Changes in bone morphology were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 34 times the clinical dose of omeprazole. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age [see Data].
Clarithromycin
Limited data from a small number of published human studies with clarithromycin use during pregnancy are insufficient to inform drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, administration of oral clarithromycin to pregnant mice, rats, rabbits, and monkeys during the period of organogenesis produced malformations in rats (cardio-vascular anomalies) and mice (cleft palate) at clinically relevant doses based on body surface area comparison. Fetal effects in mice, rats, and monkeys (e.g., reduced fetal survival, body weight, body weight gain) and implantation losses in rabbits were generally considered to be secondary to maternal toxicity (see Animal Data).
Amoxicillin
Available data from published epidemiologic studies and pharmacovigilance case reports over several decades with amoxicillin use have not established drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). No adverse developmental effects were observed in animal reproduction studies with administration of amoxicillin to pregnant mice and rats at doses up to 3 and 6 times the recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
Omeprazole
Reproductive studies conducted with omeprazole in rats at oral doses up to 138 mg/kg/day (about 34 times an oral human dose of 40 mg based on body surface area comparison) and in rabbits at doses up to 69.1 mg/kg/day (about 34 times an oral human dose of 40 mg) during organogenesis did not disclose any evidence for a teratogenic potential of omeprazole. In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg) administered during organogenesis produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138.0 mg/kg/day (about 3.4 to 34 times an oral human doses of 40 mg), administered prior to mating through the lactation period.
Clarithromycin
Animal reproduction studies were conducted in mice, rats, rabbits, and monkeys with oral and intravenously administered clarithromycin. In pregnant mice, clarithromycin was administered during organogenesis (gestation day [GD] 6 to 15) at oral doses of 15, 60, 250, 500, or 1000 mg/kg/day. Reduced body weight observed in dams at 1000 mg/kg/day (3 times the maximum recommended human dose [MRHD]) resulted in reduced survival and body weight of the fetuses. At ≥ 500 mg/kg/day, increases in the incidence of post-implantation loss and cleft palate in the fetuses were observed. No adverse developmental effects were observed in mice at ≤ 250 mg/kg/day (≤ 1 times MRHD comparison).
In pregnant Sprague Dawley rats, clarithromycin administered during organogenesis (GD 6 to 15) at oral doses of 15, 50, or 150 mg/kg/day resulted in reduced body weight and food consumption in dams at 150 mg/kg/day. Reductions in body weight and food consumption was observed in dams at 150 mg/kg/day. Increased resorptions and reduced body weight of the fetuses at this dose were considered secondary to maternal toxicity. Additionally, at 150 mg/kg/day (1 times MRHD), a low incidence of cardiovascular anomalies (complete situs inversus, undivided truncus, IV septal defect) was observed in the fetuses. Clarithromycin did not cause adverse developmental effects in rats at 50 mg/kg/day (0.3 times MRHD comparison). Intravenous dosing of clarithromycin during organogenesis in rats (GD 6 to 15) at 15, 50, or 160 mg/kg/day was associated with maternal toxicity (reduced body weight, body-weight gain, and food consumption) at 160 mg/kg/day but no evidence of adverse developmental effects at any dose (≤ 1 times MRHD comparison).
In pregnant Wistar rat, clarithromycin was administered during organogenesis (GD 7 to 17) at oral doses of 10, 40, or 160 mg/kg/day. Reduced body weight and food consumption were observed in dams at 160 mg/kg/day but there was no evidence of adverse developmental effects at any dose (≤ 1 times MRHD comparison).
In pregnant rabbits, clarithromycin administered during organogenesis (GD 6 to 18) at oral doses of 10, 35, or 125 mg/kg/day resulted in reduced maternal food consumption and decreased body weight at the highest dose, with no evidence of any adverse developmental effects at any dose (≤ 2 times MRHD comparison). Intravenously administered clarithromycin to pregnant rabbits during organogenesis (GD 6 to 18) in rabbits at 20, 40, 80, or 160 mg/kg/day (≥ 0.3 times MRHD comparison) resulted in maternal toxicity and implantation losses at all doses.
In pregnant monkeys, clarithromycin administered (GD 20 to 50) at oral doses of 35 or 70 mg/kg/day resulted in dose-dependent emesis, poor appetite, fecal changes, and reduced body weight in dams at all doses (≥ 0.5 times MRHD comparison). Growth retardation in 1 fetus at 70 mg/kg/day was considered secondary to maternal toxicity. There was no evidence of primary drug related adverse developmental effects at any dose tested.
In a reproductive toxicology study in rats administered oral clarithromycin late in gestation through lactation (GD 17 to post-natal day 21) at doses of 10, 40, or 160 mg/kg/day (≤ 1 times MRHD comparison), reductions in maternal body weight and food consumption were observed at 160 mg/kg/day. Reduced body-weight gain observed in offspring at 160 mg/kg/day was considered secondary to maternal toxicity. No adverse developmental effects were observed with clarithromycin at any dose tested.
Human Data
Omeprazole
Four published epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, from 1995 to 99, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed in utero to omeprazole that had any malformation, low birth weight, low Apgar score, or hospitalization was similar to the number observed in this population. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole-exposed infants than the expected number in this population.
A population-based retrospective cohort study covering all live births in Denmark from 1996 to 2009, reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837,317 live births whose mothers did not use any proton pump inhibitor. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any proton pump inhibitor during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2-blocker, or were unexposed was 3.6%, 5.5%, and 4.1% respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% with first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease-paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Amoxicillin
While available studies cannot definitively establish the absence of risk, published epidemiological data and post-marketing case reports have not reported a consistent association with amoxicillin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when amoxicillin was used during pregnancy. Available studies have methodologic limitations, including small sample size, retrospective data collection, under-capture of non-live births, exposure misclassification and inconsistent comparator groups.
8.2 Lactation
Risk Summary
Based on limited human data, clarithromycin and its active metabolite 14-OH clarithromycin are present in human milk at less than 2% of the maternal weight-adjusted dose (see Data). In a separate observational study, reported adverse effects on breast-fed children (rash, diarrhea, loss of appetite, somnolence) were comparable to amoxicillin (see Data). No data are available to assess the effects of clarithromycin or 14-OH clarithromycin on milk production.
Limited data from a single case report suggest omeprazole may be present in human milk. There are no clinical data on the effects of omeprazole on the breastfed infant or on milk production.
Data from a published clinical lactation study indicate that amoxicillin is present in human milk. Published adverse effects with amoxicillin exposure in the breast-fed infant include diarrhea. There are no data on the effects of amoxicillin on milk production.
The development and health benefits of breastfeeding should be considered along with the mother's clinical need for omeprazole or clarithromycin or amoxicillin and any potential adverse effects on the breast-fed child from clarithromycin or from the underlying maternal condition.
Data
Clarithromycin
Serum and milk samples were obtained after 3 days of treatment, at steady state, from one published study of 12 lactating women who were taking clarithromycin 250 mg orally twice daily. Based on the limited data from this study, and assuming milk consumption of 150 mL/kg/day, an exclusively human milk fed infant would receive an estimated average of 136 mcg/kg/day of clarithromycin and its active metabolite, with this maternal dosage regimen. This is less than 2% of the maternal weight-adjusted dose (7.8 mg/kg/day, based on the average maternal weight of 64 kg), and less than 1% of the pediatric dose (15 mg/kg/day) for children greater than 6 months of age.
A prospective observational study of 55 breastfed infants of mothers taking a macrolide antibacterial (6 were exposed to clarithromycin) were compared to 36 breastfed infants of mothers taking amoxicillin. Adverse reactions were comparable in both groups. Adverse reactions occurred in 12.7% of infants exposed to macrolides and included rash, diarrhea, loss of appetite, and somnolence.
8.3 Females and Males of Reproductive Potential
Infertility
Clarithromycin
Administration of clarithromycin resulted in testicular atrophy in rats, dogs and monkeys [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of OMECLAMOX-PAK for pediatric patients with H. pylori have not been established.
8.5 Geriatric Use
Omeprazole
Omeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the U.S. and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Pharmacokinetic studies have shown the elimination rate was somewhat decreased in the elderly and bioavailability was increased. The plasma clearance of omeprazole was 250 mL/min (about half that of young volunteers) and its plasma half-life averaged one hour, about twice that of young healthy volunteers. However, no dosage adjustment is necessary in the elderly [see Clinical Pharmacology (12.3)].
Clarithromycin
In a steady-state study in which healthy elderly subjects (age 65 to 81 years old) were given 500 mg every 12 hours, the maximum serum concentrations and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults. These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse events when compared to younger patients.
Amoxicillin
An analysis of clinical studies of amoxicillin was conducted to determine whether subjects aged 65 and over respond differently from younger subjects. Of the 1811 subjects treated with amoxicillin, 85% were < 60 years old, 15% were ≥ 61 years old and 7% were ≥ 71 years old. This analysis and other reported clinical experience have not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
In the presence of severe renal impairment with or without coexisting hepatic impairment, prolonged dosing intervals for the clarithromycin component may be appropriate.
8.7 Hepatic Impairment
It is recommended to avoid the use of OMECLAMOX-PAK in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
8.8 Asian Patients
It is recommended to avoid the use of OMECLAMOX-PAK in Asian patients unless it is deemed that the benefits outweigh the risks [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In case of an overdose, patients should contact a physician, poison control center, or emergency room. There is neither a pharmacologic basis nor data suggesting an increased toxicity of the combination compared to individual components.
As with the management of any overdose, the possibility of multiple drug ingestion should be considered. For current information on treatment of any drug overdose, contact your local Poison Control Center at 1-800-222-1222.
Omeprazole:
Reports have been received of overdosage with omeprazole in humans. Doses ranged up to 2400 mg (120 times the usual recommended clinical dose). Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, vomiting, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience [see Adverse Reactions (6.3)]. Symptoms were transient, and no serious clinical outcome has been reported when omeprazole was taken alone. No specific antidote for omeprazole overdosage is known. Omeprazole is extensively protein bound and is, therefore, not readily dialyzable. In the event of overdosage, treatment should be symptomatic and supportive.
Single oral doses of omeprazole at 1350, 1339, and 1200 mg/kg were lethal to mice, rats, and dogs, respectively. Animals given these doses showed sedation, ptosis, tremors, convulsions, and decreased activity, body temperature, and respiratory rate and increased depth of respiration.
Clarithromycin:
Overdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea.
Adverse reactions accompanying overdosage should be treated by the prompt elimination of unabsorbed drug and supportive measures. As with other macrolides, clarithromycin serum concentrations are not expected to be appreciably affected by hemodialysis or peritoneal dialysis.
Amoxicillin:
In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required. If the overdosage is very recent and there is no contraindication, an attempt at emesis or other means of removal of drug from the stomach may be performed. A prospective study of 51 pediatric patients at a poison-control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying1.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin. Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria. Renal impairment appears to be reversible with cessation of drug administration. High blood concentrations may occur more readily in patients with impaired renal function because of decreased renal clearance of amoxicillin. Amoxicillin can be removed from circulation by hemodialysis.
-
11 DESCRIPTION
OMECLAMOX-PAK consists of a pack of ten individual daily administration cards, each card containing two omeprazole delayed-release 20 mg capsules, USP, two clarithromycin 500 mg tablets, USP, and four amoxicillin 500 mg capsules, USP, for oral administration.
Omeprazole Delayed-Release Capsules, USP
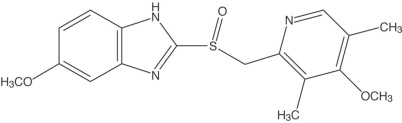
The active ingredient in omeprazole delayed-release capsules is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl- 2-pyridinyl) methyl] sulfinyl]1H-benzimidazole, a proton pump inhibitor that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42. The structural formula is:
Omeprazole is a white to off-white crystalline powder that melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol, and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
Each omeprazole delayed-release capsule contains 20 mg of omeprazole in the form of enteric-coated granules with the following inactive ingredients: crospovidone, hypromellose, lactose, magnesium stearate, mannitol, meglumine, methacrylic acid copolymer, poloxamer, povidone and triethyl acetate. The capsule shells contain: D&C Red #28, FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, yellow iron oxide, gelatin, silicon dioxide, sodium lauryl sulfate and titanium dioxide. Imprinting ink contains: D&C Yellow No. 10 aluminum lake, FD&C Blue No. 1 aluminum lake, FD&C Blue No. 2 aluminum lake, FD&C Red No. 40 aluminum lake, n-butyl alcohol, pharmaceutical glaze, propylene glycol, SDA-3A alcohol and synthetic black iron oxide.
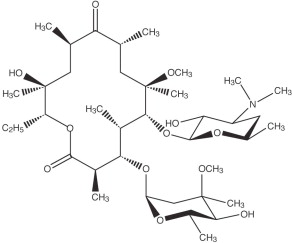
Clarithromycin Tablets, USP
Clarithromycin is a semi-synthetic macrolide antimicrobial. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. Clarithromycin has the following structural formula:
Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water. Each tablet for oral administration contains 500 mg of clarithromycin and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, Opadry II (White), povidone, stearic acid, and talc. Opadry II (White) contains hypromellose, polyethylene glycol, polydextrose, titanium dioxide and triacetin.
Amoxicillin Capsules, USP:
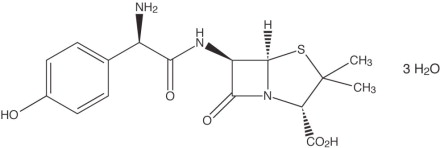
Amoxicillin, a semisynthetic penicillin class antibacterial, is an analogue of ampicillin, with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms. Chemically it is (2S, 5R, 6R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl)acetamido]- 3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0] heptane-2-carboxylic acid trihydrate. Its empirical formula is C16H19N3O5S •3H2O with a molecular weight of 419.45. Amoxicillin has the following structural formula:
Amoxicillin capsules contain amoxicillin trihydrate equivalent to 500 mg of amoxicillin. Amoxicillin capsules USP also contain magnesium stearate and sodium lauryl sulfate. The capsule shell contains D&C Red No. 33, FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, sodium lauryl sulfate and titanium dioxide. Each 500 mg capsule contains up to 0.0052 mEq (0.119 mg) of sodium.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Omeprazole is an antisecretory drug whereas clarithromycin and amoxicillin are antibacterial drugs [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Pharmacokinetics when all three of the OMECLAMOX-PAK components were coadministered has not been studied. Studies have shown the low risk of clinically significant interactions of omeprazole and amoxicillin or omeprazole and clarithromycin when administered together. There is no information about the gastric mucosal concentrations of omeprazole, clarithromycin and amoxicillin after administration of these drugs concomitantly. The systemic pharmacokinetic information presented below is based on studies in which each product was administered alone, or in combination of two components.
Omeprazole Delayed-Release Capsules, USP
Absorption and Distribution
Omeprazole delayed-release capsules contain an enteric-coated granule formulation of omeprazole (because omeprazole is acid- labile), so that absorption of omeprazole begins only after the granules leave the stomach. Absorption is rapid, with peak plasma concentrations of omeprazole occurring within 0.5 to 3.5 hours. Peak plasma concentrations of omeprazole and AUC are approximately proportional to doses up to 40 mg, but because of a saturable first-pass effect, a greater than linear response in peak plasma concentration and AUC occurs with doses greater than 40 mg. Absolute bioavailability (compared with intravenous administration) is about 30-40% at doses of 20-40 mg, due in large part to presystemic metabolism. In healthy subjects the plasma half-life is 0.5 to 1 hour, and the total body clearance is 500-600 mL/min.
The bioavailability of omeprazole increases slightly upon repeated administration of omeprazole delayed-release capsules.
Omeprazole delayed-release capsules 40 mg was bioequivalent when administered with and without applesauce. However, omeprazole delayed-release capsules 20 mg was not bioequivalent when administered with and without applesauce. When administered with applesauce, a mean 25% reduction in Cmax was observed without a significant change in AUC for omeprazole delayed-release capsules 20 mg. The clinical relevance of this finding is unknown. Protein binding is approximately 95%.
Metabolism and Excretion
Among those females aged 18 to 44 years of age, 22.4% were diagnosed with skin infections and 7.6% had pneumonia.
Geriatric Patients
The elimination rate of omeprazole was somewhat decreased in the elderly, and bioavailability was increased. Omeprazole was 76% bioavailable when a single 40 mg oral dose of omeprazole (buffered solution) was administered to healthy elderly volunteers, versus 58% in young volunteers given the same dose. Nearly 70% of the dose was recovered in urine as metabolites of omeprazole and no unchanged drug was detected. The plasma clearance of omeprazole was 250 mL/min (about half that of young volunteers) and its plasma half-life averaged one hour, about twice that of young healthy volunteers.
Hepatic Impairment
In patients with chronic hepatic disease, the bioavailability of omeprazole increased to approximately 100% compared with an IV dose, reflecting decreased first-pass effect, and the plasma half-life of the drug increased to nearly 3 hours compared with the half-life in normals of 0.5-1 hour. Plasma clearance averaged 70 mL/min, compared with a value of 500-600 mL/min in normal subjects. It is recommended to avoid the use of OMECLAMOX-PAK in patients with hepatic impairment.
Renal Impairment
In patients with chronic renal impairment, whose creatinine clearance ranged between 10 and 62 mL/min/1.73m2, the disposition of omeprazole was very similar to that in healthy volunteers, although there was a slight increase in bioavailability. Because urinary excretion is a primary route of excretion of omeprazole metabolites, their elimination slowed in proportion to the decreased creatinine clearance. No dose reduction is necessary in patients with renal impairment.
Asian Patients
In pharmacokinetic studies of single 20 mg omeprazole doses, an increase in AUC of approximately four-fold was noted in Asian subjects compared with Caucasians. It is recommended to avoid the use of OMECLAMOX-PAK in Asian patients unless it is deemed that the benefits outweigh the risks.
Clarithromycin Tablets, USP
Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250 mg clarithromycin tablets was approximately 50%. For a single 500 mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability. Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly increase the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, clarithromycin tablets may be given without regard to food.
In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 3 to 4 μg/mL with a 500 mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was 5 to 7 hours with 500 mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended dose of 500 mg administered every 8 to 12 hours. With a 500 mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is up to 1 μg/mL, and its elimination half-life is about 7 to 9 hours. The steady-state concentration of this metabolite is generally attained within 3 to 4 days.
After a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is approximately 30%. The renal clearance of clarithromycin approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with a 500 mg tablet administered every 12 hours.
The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
The pharmacokinetics of clarithromycin was altered in subjects with impaired renal function. In the presence of severe renal impairment with or without coexisting hepatic impairment, prolonged dosing intervals for clarithromycin may be appropriate.
Amoxicillin Capsules, USP
Amoxicillin is stable in the presence of gastric acid and may be given without regard to meals. It is rapidly absorbed after oral administration. It diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid, except when meninges are inflamed. The half-life of amoxicillin is 61.3 minutes. Most of the amoxicillin is excreted unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid. In blood serum, amoxicillin is approximately 20% protein- bound.
Orally administered doses of 500 mg amoxicillin capsules result in average peak blood concentrations 1 to 2 hours after administration in the range of 5.5 μg /mL to 7.5 μg /mL. Detectable serum concentrations are observed up to 8 hours after an orally administered dose of amoxicillin. Approximately 60% of an orally administered dose of amoxicillin is excreted in the urine within 6 to 8 hours.
Combination Therapy of Omeprazole with Antimicrobials:
Omeprazole 40 mg daily was given in combination with clarithromycin 500 mg every 8 hours to healthy adult male subjects. The steady-state plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T1/2 increases of 30%, 89% and 34% respectively) by the concomitant administration of clarithromycin. The observed increases in omeprazole plasma concentration were associated with the following pharmacological effects. The mean 24-hour gastric pH value was 5.2 when omeprazole was administered alone and 5.7 when coadministered with clarithromycin.
The plasma concentrations of clarithromycin and 14-hydroxy-clarithromycin were increased by the concomitant administration of omeprazole. For clarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27% greater, and the mean AUC0-8 was 15% greater when clarithromycin was administered with omeprazole than when clarithromycin was administered alone. Similar results were seen for 14-hydroxy-clarithromycin, the mean Cmax was 45% greater, the mean Cmin was 57% greater, and the mean AUC0-8 was 45% greater. Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole.
Table 1 Mean ± SD Clarithromycin Tissue Concentrations 2 Hours after Dose Tissue Clarithromycin (μg/g) Clarithromycin + Omeprazole (μg/g) Antrum 10.48 ± 2.01 (n = 5) 19.96 ± 4.71 (n = 5) Fundus 20.81 ± 7.64 (n = 5) 24.25 ± 6.37 (n = 5) Mucus 4.15 ± 7.74 (n = 4) 39.29 ± 32.79 (n = 4) Drug Interactions
Antiretroviral Drugs and Omeprazole
The clinical importance and the mechanisms behind interactions between omeprazole and antiretroviral drugs are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the antiretroviral drug. Other possible interaction mechanisms are via inhibition of CYP2C19.
Following multiple doses of nelfinavir (1250 mg twice daily) and omeprazole (40 mg once daily), AUC of nelfinavir and the M8 metabolite was decreased by 36% and 92%, Cmax by 37% and 89% and Cmin by 39% and 75%.
Following multiple doses of atazanavir (400 mg once daily) and omeprazole (40 mg once daily 2 hours before atazanavir), AUC of atazanavir was decreased by 94%, Cmax by 96%, and Cmin by 95%. Concomitant administration with omeprazole and atazanavir is not recommended.
Saquinavir serum AUC, Cmax and Cmin increased by 82%, 75% and 106%, respectively, following multiple dosing of saquinavir/ritonavir (1000/100 mg) twice daily for 15 days with omeprazole 40 mg once daily coadministered days 11 to 15 [see Drug Interactions (7.6)].
Cilostazol and Omeprazole
Omeprazole acts as an inhibitor of CYP2C19. Omeprazole, given in doses of 40 mg daily for one week to 20 healthy subjects in crossover study, increased Cmax and AUC of cilostazol by 18% and 26% respectively. Cmax and AUC of one of its active metabolites, 3,4-dihydro-cilostazol, which has 4-7 times the activity of cilostazol, were increased by 29% and 69% respectively. Co-administration of cilostazol with omeprazole is expected to increase concentrations of cilostazol and its above mentioned active metabolite. Therefore a dose reduction of cilostazol from 100 mg b.i.d. to 50 mg b.i.d. should be considered [see Drug Interactions (7.7)].
Theophylline and Clarithromycin
Theophylline is metabolized by CYP1A2 and CYP3A4. Clarithromycin will increase theophylline plasma concentrations when it is administered concomitantly. In two studies in which theophylline was administered with clarithromycin (theophylline sustained- release formulation dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg every 12 hours clarithromycin), the steady-state Cmax, Cmin, and AUC of theophylline increased about 20%. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range [see Drug Interactions (7.9)].
Voriconazole and Omeprazole
Voriconazole is an inhibitor of CYP2C19, CYP2C9, and CYP3A4. Coadministration of voriconazole and omeprazole will increase omeprazole plasma exposure. When voriconazole (400 mg every 12 hours x 1 day, then 200 mg x 6 days) was given with omeprazole (40 mg once daily x 7 days) to healthy subjects, it significantly increased the steady-state Cmax and AUC0-24 of omeprazole, an average of 2 times (90% CI: 1.8, 2.6) and 4 times (90% CI: 3.3, 4.4) respectively as compared to when omeprazole was given without voriconazole. Dose adjustment of omeprazole is not normally required.
Mycophenolate mofetil
Administration of omeprazole 20 mg twice daily for 4 days and a single 1000 mg dose of mycophenolate mofetil approximately one hour after the last dose of omeprazole to 12 healthy subjects in a cross-over study resulted in a 52% reduction in the Cmax and 23% reduction in the AUC of mycophenolic acid.
12.4 Microbiology
Mechanism of Action
Omeprazole, an antisecretory drug with the substituted benzimidazoles, suppresses gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-dependent and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Omeprazole can also exhibit anti-bacterial activity depending on the culture conditions. Animal studies indicate that after rapid disappearance from plasma, omeprazole can be found within the gastric mucosa for a day or more.
Clarithromycin exerts its antibacterial activity by binding to the 50S ribosomal subunit of susceptible microorganisms resulting in inhibition of protein synthesis.
Amoxicillin acts through the inhibition of biosynthesis of cell wall mucopeptide.
Triple therapy with omeprazole, clarithromycin and amoxicillin has been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections as indicated [see Indications and Usage (1)].
Interactions with Other Antibacterial Drugs
In vitro studies show that chloramphenicol, macrolides, sulfonamides, and tetracyclines may interfere with bactericidal effects of penicillin; however, the clinical significance of this interaction is not well documented.
Drug Resistance
Helicobacter pylori Pretreatment Resistance
Clarithromycin pretreatment resistance rates were 9.3% (41/439) in omeprazole/clarithromycin/amoxicillin triple therapy studies [see Clinical Studies (14.1)].
Amoxicillin pretreatment susceptible isolates (≤ 0.25 μg/mL) were found in 99.3% (436/439) of the patients in the omeprazole/clarithromycin/amoxicillin triple therapy studies [see Clinical Studies (14)].)
Effects on Gastrointestinal Microbial Ecology:
Decreased gastric acidity due to any means including proton pump inhibitors, increases gastric counts of bacteria normally present in the gastrointestinal tract. Treatment with proton pump inhibitors may lead to slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Omeprazole:
Carcinogenicity
In two 24-month carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44.0 and 140.8 mg/kg/day (about 0.7 to 57 times a human dose of 20 mg/day, as expressed on a body surface area basis) produced gastric ECL cell carcinoids in a dose-related manner in both males and females; the incidence of this effect was markedly higher in female rats, which had higher blood concentrations of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 6 times a human dose of 20 mg/day, based on body surface area) for one year, and then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of one year (94% treated vs. 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs. 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for two years. For this strain of rat no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret.
In a 52-week toxicity study in Sprague-Dawley rats, brain astrocytomas were found in a small number of males that received omeprazole at dose levels of 0.4, 2, and 16 mg/kg/day (about 0.2 to 6.5 times the human dose on a body surface area basis). No astrocytomas were observed in female rats in this study or in males or females from a 2-year carcinogenicity study in Sprague-Dawley rats at the high dose of 140.8 mg/kg/day (about 57 times the human dose on a body surface area basis). A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive. A 26-week p53 (+/–) transgenic mouse carcinogenicity study was not positive.
Genotoxicity
Omeprazole was positive for clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in one of two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames test, an in vitro mouse lymphoma cell forward mutation assay, and an in vivo rat liver DNA damage assay.
Clarithromycin:
Carcinogenicity
Carcinogencity studies have not been conducted with clarithromycin.
The following in vitro mutagenicity tests have been conducted with clarithromycin:
- Bacterial reverse-mutation test (Ames test)
- Salmonella/Mammalian Microsomes Test
- Bacterial Induced Mutation Frequency Test
- In Vitro Chromosome Aberration Test
- Rat Hepatocyte DNA Synthesis Assay
- Mouse Lymphoma Assay
- Mouse Dominant Lethal Study
- Mouse Micronucleus Test
All tests had negative results except the in vitro chromosome aberration test which was positive in one test and negative in another.
Impairment of Fertility
Fertility and reproduction studies have shown that daily doses of clarithromycin up to 160 mg/kg/day to male and female rats caused no adverse effects on the estrous cycle, fertility, parturition, or number and viability of offspring. Plasma level sin rats after 150 mg/kg/day were twice the human serum levels.
Testicular atrophy occurred in rats at doses 7 times, in dogs at doses 3 times, and in monkeys at doses 8 times the maximum human daily dose (on a body surface area basis).
-
14 CLINICAL STUDIES
14.1 H. pylori associated Duodenal Ulcer Disease
Three U.S., randomized, double-blind clinical studies in patients with H. pylori infection and duodenal ulcer disease (n = 558) compared omeprazole plus clarithromycin plus amoxicillin with clarithromycin plus amoxicillin. Two studies (1 and 2) were conducted in patients with an active duodenal ulcer, and the other study (3) was conducted in patients with a history of a duodenal ulcer in the past 5 years but without an ulcer present at the time of enrollment. The dose regimen in the studies was omeprazole 20 mg twice daily plus clarithromycin 500 mg twice daily plus amoxicillin 1 g twice daily for 10 days; or clarithromycin 500 mg twice daily plus amoxicillin 1 g twice daily for 10 days. In studies 1 and 2, patients who took the omeprazole regimen also received an additional 18 days of omeprazole 20 mg once daily. Endpoints studied were eradication of H. pylori and duodenal ulcer healing (studies 1 and 2 only). H. pylori status was determined by CLOtest®, histology and culture in all three studies. For a given patient, H. pylori was considered eradicated if at least two of these tests were negative and none was positive.
The combination of omeprazole plus clarithromycin plus amoxicillin, was effective in eradicating H. pylori.
Table 2 Per-Protocol and Intent-to-Treat H. pylori Eradication Rates - % of Patients Cured [95% Confidence Interval] †Patients were included in the analysis if they had confirmed duodenal ulcer disease (active ulcer, studies 1 and 2; history of ulcer within 5 years, study 3) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. The impact of eradication on ulcer recurrence has not been assessed in patients with a past history of ulcer.
‡Patients were included in the analysis if they had documented H. pylori infection at baseline and had confirmed duodenal ulcer disease. All dropouts were included as failures of therapy.
*(p < 0.05) versus clarithromycin plus amoxicillin.
Per-Protocol and Intent-to-Treat H. pylon Eradication Rates
% of Patients Cured [95% Confidence Interval]omeprazole + chlarithromycin + amoxicillin chlarithromycin + amoxicillin Per-Protocol† Intent-to-Treat‡ Per-Protocol† Intent-to-Treat‡ Study 1 *77 [64, 86]
(n = 64)*69 [57, 79]
(n = 80)43 [31, 56]
(n = 67)37 [27, 38]
(n = 84)Study 2 *78 [67, 88]
(n = 65)*73 [61, 82]
(n = 77)41 [29, 54]
(n = 68)36 [26, 47]
(n = 83)Study 3 *90 [80, 96]
(n = 69)*83 [74, 91]
(n = 84)33 [24, 44]
(n = 93)32 [23, 42]
(n = 99) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OMECLAMOX-PAK is supplied in a carton containing ten individual daily administration cards. Each card contains the morning dose and the evening dose of the following three drugs:
Omeprazole Delayed-Release Capsules, USP, 20 mg
- Two opaque hard gelatin lavender and grey capsules, with ‘R 158’ and ‘OMEPRAZOLE 20 mg’ imprinted on the capsules in black ink, containing off-white to pale-yellow, elliptical spherical pellets.
Clarithromycin Tablets, USP, 500 mg
- Two white, biconvex beveled-edge capsule-shaped coated tablets debossed with ‘54 312’ on one side and plain on the other side.
Amoxicillin Capsules, USP, 500 mg
- Four opaque hard gelatin yellow capsules, marked ’GG849’. Each capsule contains amoxicillin trihydrate equivalent to 500 mg amoxicillin.
NDC 66220-422-02 Carton containing 10 daily administration cards NDC 66220-422-01 Daily administration card Store at controlled room temperature between 20°C and 25°C (68°F and 77°F). Protect from light and moisture.
-
17 PATIENT COUNSELING INFORMATION
Administration
Inform patients that each dose of OMECLAMOX-PAK contains four pills: one opaque lavender/grey capsule (omeprazole), one white tablet (clarithromycin) and two opaque yellow capsules (amoxicillin).
Take each dose of four pills in the morning and four pills in the evening before eating a meal, for 10 days. Capsules and tablets should not be crushed or chewed, and should be swallowed whole [see Dosage and Administration (2)].
Embryo-Fetal Toxicity
Advise females of reproductive potential that that if pregnancy occurs while taking this OMECLAMOX-PAK, there is a potential hazard to the fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Drug Interactions
Patients should be advised to report to their doctor the use of any other medications while taking OMECLAMOX-PAK [see Drug Interactions (7)].
The simultaneous administration of any of the following drugs with OMECLAMOX-PAK may result in clinically significant adverse reactions or even death:
- Colchicine
- Ergotamine/dihydroergotamine
- Pimozide
- Lurasidone
- Antiarrhythmic drugs (e.g., quinidine, disopyramide)
- Digoxin
- Anticoagulants (e.g., warfarin)
- Atazanavir
- Nelfinavir
- Saquinavir
- Cilostazol
- Tacrolimus
- Theophylline
- Carbamazepine
- Sildenafil
- HMG-CoA reductase inhibitors (also known as statins)
- Triazolobenzodiazepines (e.g., triazolam and alprazolam) and related benzodiazepines (e.g., midazolam)
- Probenecid
- Drugs for which gastric pH can affect bioavailability
Diarrhea
Advise patients that diarrhea is a common problem caused by omeprazole and antibiotics that usually ends when the drug is discontinued. Sometimes after starting treatment, patients can develop severe diarrhea with watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the drug. If this occurs, patients should contact a physician as soon as possible [see Warnings and Precautions (5.4)].
Acute Tubulointerstitial Nephritis
Advise the patient or caregiver to call the patient's healthcare provider immediately if they experience signs and/or symptoms associated with acute tubulointerstitial nephritis [see Warnings and Precautions (5.7)].
Cutaneous or Systemic Lupus
Advise patients to report any symptoms associated with cutaneous or systemic lupus erythematosus [see Warnings and Precautions (5.8)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including OMECLAMOX-PAK should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When OMECLAMOX-PAK is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by OMECLAMOX-PAK or other antibacterial drugs in the future.
Severe Cutaneous Adverse Reactions
Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking OMECLAMOX-PAK immediately and promptly report the first signs or symptoms of skin rash, mucosal lesions, or any other sign of hypersensitivity [see WARNINGS AND PRECAUTIONS (5.2)].
OMECLAMOX-PAK is manufactured for CUMBERLAND PHARMACEUTICALS INC., Nashville, TN 37203.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
OMECLAMOX-PAK
omeprazole, clarithromycin, amoxicillin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66220-422 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66220-422-02 10 in 1 CARTON 04/27/2012 1 1 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 2 NDC:66220-422-01 1 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 04/27/2012 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 Part 2 2 Part 3 4 Part 1 of 3 OMEPRAZOLE
omeprazole capsule, delayed releaseProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEPRAZOLE (UNII: KG60484QX9) (OMEPRAZOLE - UNII:KG60484QX9) OMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 2S7830E561) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) PEG/PPG-30/160 COPOLYMER (UNII: 68T8I45V23) POVIDONE (UNII: FZ989GH94E) ETHYL ACETATE (UNII: 76845O8NMZ) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color GRAY (lavendar and gray) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code R;158;Omeprazol;20;mg Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050824 04/27/2012 Part 2 of 3 CLARITHROMYCIN
clarithromycin tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLARITHROMYCIN (UNII: H1250JIK0A) (CLARITHROMYCIN - UNII:H1250JIK0A) CLARITHROMYCIN 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape OVAL (biconvex, beveled edge capsule shaped) Size 19mm Flavor Imprint Code 54;312 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050824 04/27/2012 Part 3 of 3 AMOXICILLIN
amoxicillin capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 500 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE (peach and orange) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code WC;731 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050824 04/27/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050824 04/27/2012 Labeler - Cumberland Pharmaceuticals Inc. (069532880)