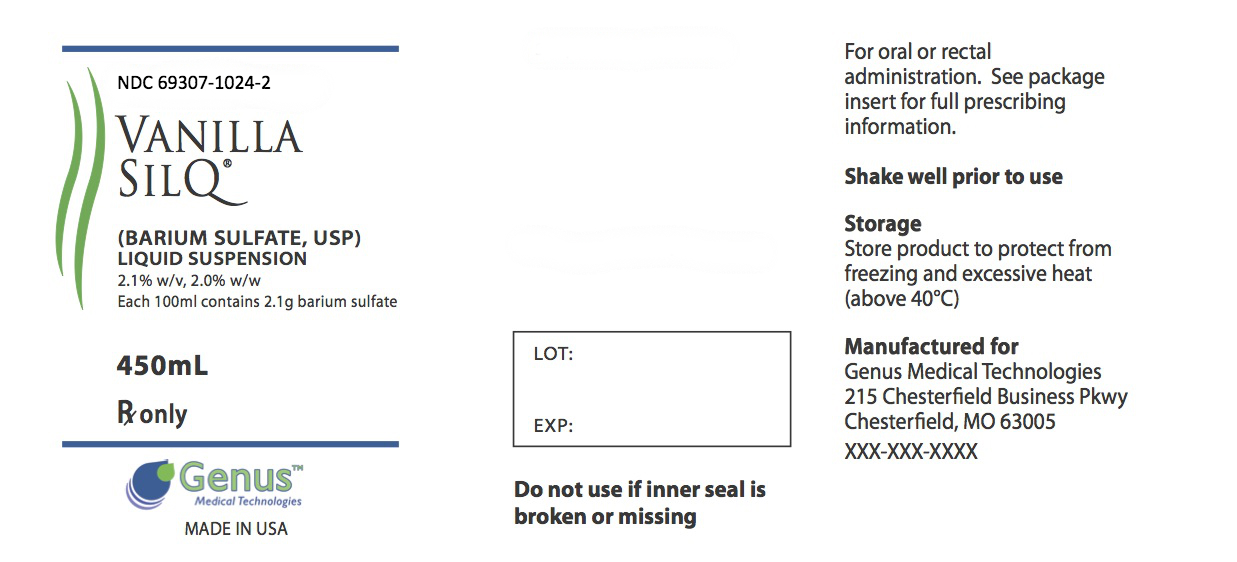
Label: VANILLA SILQ- barium sulfate suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 69307-1024-2 - Packager: Genus Medical Technologies, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONDESCRIPTION: SILQ™ is a barium sulfate suspension 2.1% w/v, 2.0% w/w for oral and rectal administration. Each 100 mL contains 2.1 g barium sulfate. Barium sulfate, due to its high molecular ...
-
INACTIVE INGREDIENTInactive Ingredients: citric acid, natural and artificial flavors, benzoic acid, suspending agent, potassium sorbate, purified water, sodium saccharin, sodium citrate, simethicone, sodium ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: Barium sulfate, due to its high molecular density is opaque to x-rays and, therefore, acts as a positive contrast agent for radiographic studies. Barium sulfate is ...
-
INDICATIONS & USAGEINDICATIONS AND USAGE: For use as a contrast agent in radiographic studies.
-
CONTRAINDICATIONSCONTRAINDICATIONS: This product should not be used in patients with known or suspected gastric or intestinal perforation, or hypersensitivity to barium sulfate or any component of this barium ...
-
WARNINGSWARNINGS: Serious adverse reactions, including death, have been reported with the administration of barium sulfate formulations and are usually associated with the technique of administration, the ...
-
PRECAUTIONSPRECAUTIONS: General: Procedures which involve the use of radiopaque contrast agents should be carried out under the direction of personnel with the requisite training and with a thorough ...
-
INFORMATION FOR PATIENTSInformation for Patients: Before using this product patients should be instructed to tell the physician ordering the procedure and the imaging technologist: if they are pregnant. if they are ...
-
DRUG INTERACTIONSDrug Interactions: The presence of barium sulfate formulations in the GI tract may alter the absorption of therapeutic agents taken concomitantly. In order to minimize any potential change in ...
-
PREGNANCYUsage in Pregnancy: Radiation is known to cause harm to the unborn fetus exposed - in utero. Therefore, radiographic procedures should only be used when, in the judgment of the physician, its ...
-
ADVERSE REACTIONSADVERSE REACTIONS: Adverse reactions accompanying the use of barium sulfate formulations are infrequent and usually mild, though severe reactions (approximately 1 in 500,000) and fatalities ...
-
OVERDOSAGEOVERDOSAGE: On rare occasions following repeated administration, severe stomach cramps, nausea, vomiting, diarrhea or constipation may occur. These are transitory in nature and are not considered ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Barium sulfate volume and method of administration are determined by individual technique, and may vary with differing patient and procedure characteristics.
-
STORAGE AND HANDLINGSTORAGE: Store product to protect from freezing and excessive heat (above 40°C).
-
PRINCIPAL DISPLAY PANELPackage Label.Principal Display Panel - 0607f088-d469-2961-e054-00144ff8d46c.jpg
-
PRINCIPAL DISPLAY PANEL
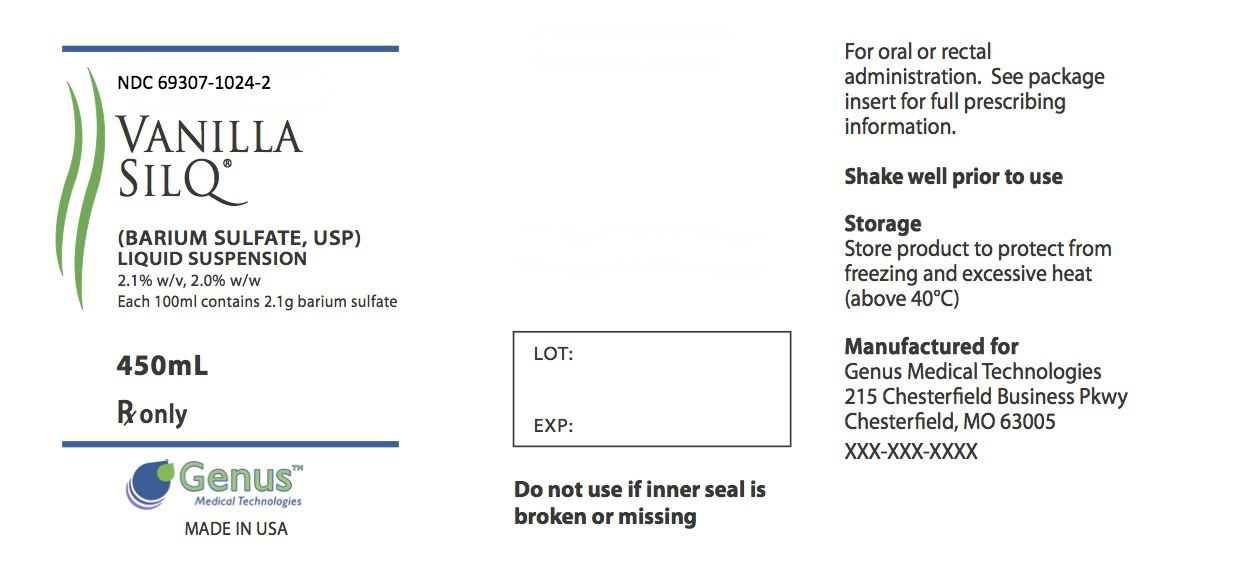
-
INGREDIENTS AND APPEARANCEProduct Information