Label: SOLU-MEDROL- methylprednisolone sodium succinate injection, powder, for solution
- NDC Code(s): 0009-0003-02, 0009-0018-20, 0009-0039-28, 0009-0039-30, view more
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONThe formulations containing benzyl alcohol should not be used in neonates. For Intravenous or Intramuscular Administration
-
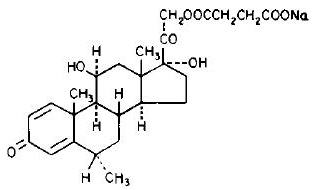
DESCRIPTIONSOLU-MEDROL Sterile Powder is an anti-inflammatory glucocorticoid, which contains methylprednisolone sodium succinate as the active ingredient. Methylprednisolone sodium succinate, USP, is the ...
-
CLINICAL PHARMACOLOGYGlucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and ...
-
INDICATIONS AND USAGEWhen oral therapy is not feasible, and the strength, dosage form, and route of administration of the drug reasonably lend the preparation to the treatment of the condition, the intravenous or ...
-
CONTRAINDICATIONSSOLU-MEDROL Sterile Powder is contraindicated: • in systemic fungal infections and patients with known hypersensitivity to the product and its constituents. The SOLU-MEDROL 40 mg presentation ...
-
WARNINGSSerious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids. Specific ...
-
PRECAUTIONSGeneral - This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial. The lowest ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported with SOLU-MEDROL or other corticosteroids: Allergic reactions: Allergic or hypersensitivity reactions, anaphylactoid reaction, anaphylaxis ...
-
OVERDOSAGETreatment of acute overdosage is by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of the corticosteroid ...
-
DOSAGE AND ADMINISTRATIONNOTE: Some of the SOLU-MEDROL formulations contain benzyl alcohol (see DESCRIPTION, WARNINGS and PRECAUTIONS, Pediatric Use) Because of possible physical incompatibilities, SOLU-MEDROL should ...
-
STORAGE CONDITIONSProtect from light. Store unreconstituted product at controlled room temperature 20° to 25°C (68° to 77°F) [see USP]. Store reconstituted solution (not further diluted) at controlled room ...
-
HOW SUPPLIEDSOLU-MEDROL Sterile Powder preserved with benzyl alcohol is available in the following packages: 500 mg (Multi-Dose Vial) 8 mL NDC 0009-0758-01 - 2 gram Vial (Single-Dose Vial) NDC ...
-
SPL UNCLASSIFIED SECTIONLAB-0161-21.0 - Revised: April 2025
-
PRINCIPAL DISPLAY PANEL - 500 mg Vial LabelNDC 0009-0758-01 - Multi-dose vial. Discard unused portion. Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 500 mg* per vial - This Package Does Not Contain ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Vial CartonNDC 0009-0758-01 - 1 Vial - Multi-dose vial. Discard unused portion. Solu-Medrol® (methylprednisolone - sodium succinate - for injection, USP) 500 mg* per vial - For Intramuscular or - Intravenous ...
-
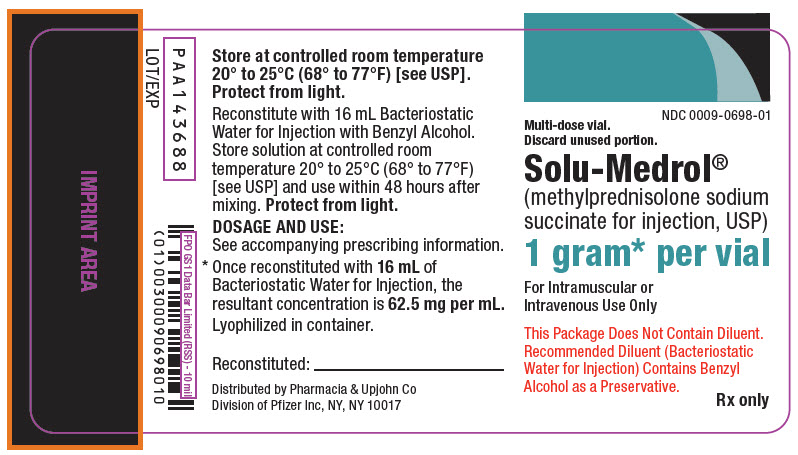
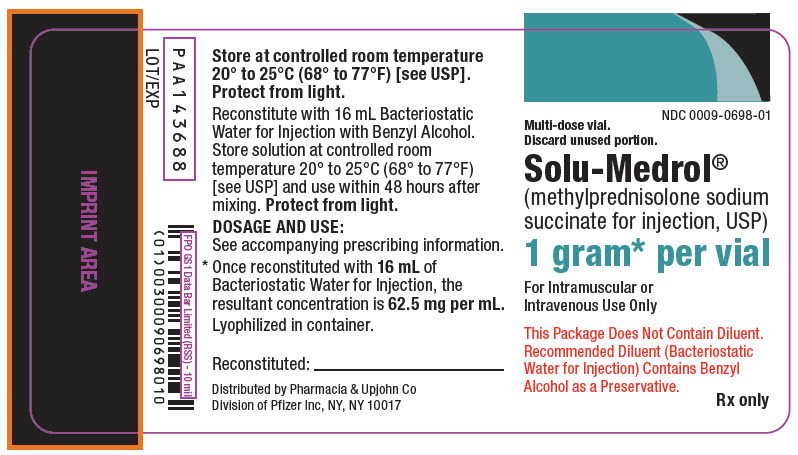
PRINCIPAL DISPLAY PANEL - 1 gram Vial LabelNDC 0009-0698-01 - Multi-dose vial. Discard unused portion. Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 1 gram* per vial - For Intramuscular or - Intravenous Use ...
-
PRINCIPAL DISPLAY PANEL - 1 gram Vial CartonNDC 0009-0698-01 - 1 Vial - Multi-dose vial. Discard unused portion. Solu-Medrol® (methylprednisolone - sodium succinate - for injection, USP) 1 gram* per vial - For Intramuscular or - Intravenous ...
-
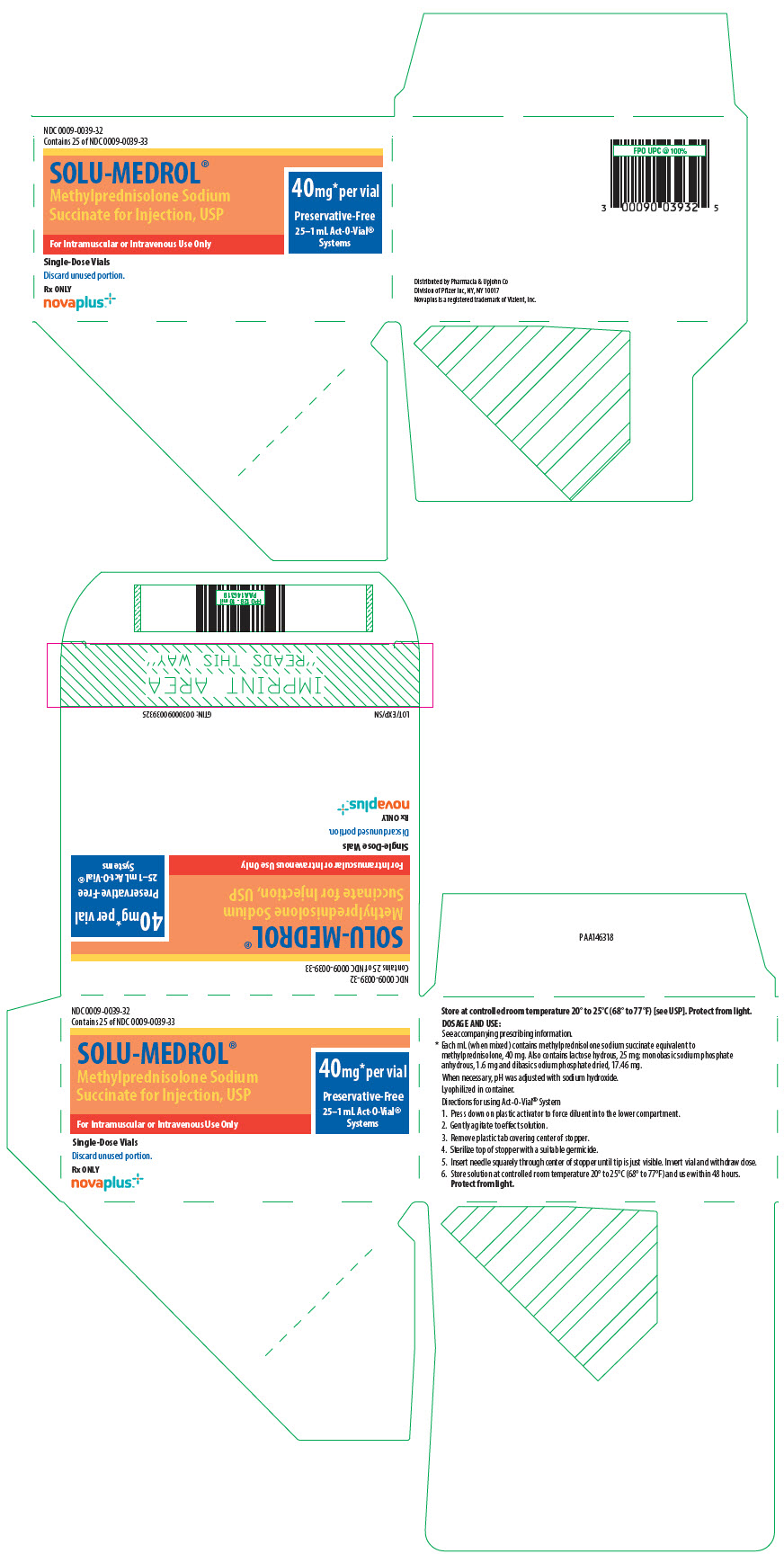
PRINCIPAL DISPLAY PANEL - 40 mg Vial LabelNDC 0009-0039-30 - 1 mL Act-O-Vial® Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 40 mg* per vial - Preservative-Free - Rx only
-
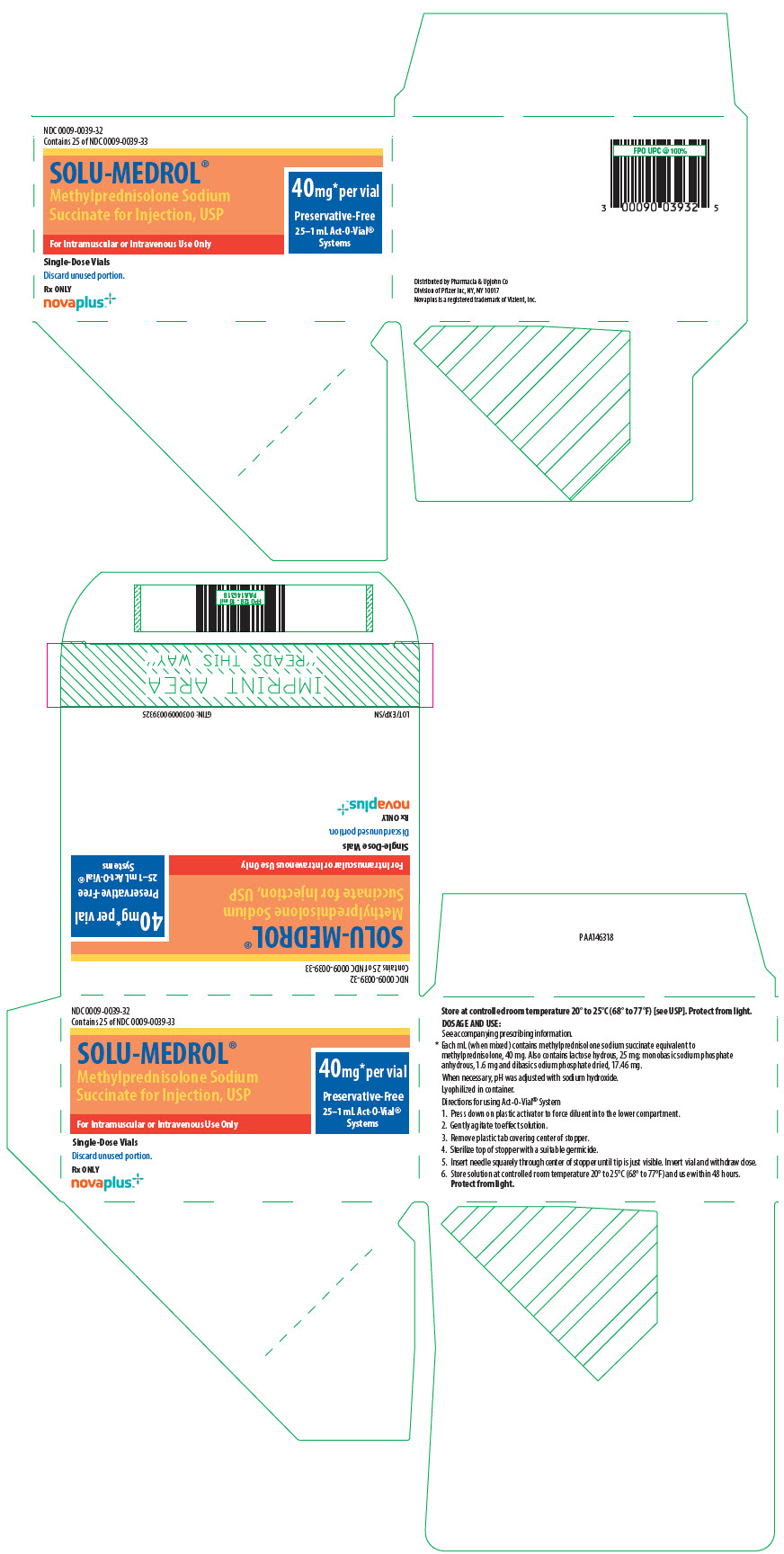
PRINCIPAL DISPLAY PANEL - 40 mg Vial CartonNDC 0009-0039-28 - Contains 25 of NDC 0009-0039-30 - Single-Dose Vial. Discard unused portion. 25–1 mL Act-O-Vial® Systems - Solu-Medrol® (methylprednisolone sodium - succinate for injection ...
-
PRINCIPAL DISPLAY PANEL - 40 mg Vial Label - Act-O-Vial SystemNDC 0009-0039-33 - Rx ONLY - SOLU-MEDROL® Methylprednisolone Sodium - Succinate for Injection, USP - Single-Dose Vial - For IM or IV Use Only - Discard unused - portion. 40 mg* per ...
-
PRINCIPAL DISPLAY PANEL - 40 mg Vial Carton - Act-O-Vial SystemNDC 0009-0039-32 - Contains 25 of NDC 0009-0039-33 - SOLU-MEDROL® Methylprednisolone Sodium - Succinate for Injection, USP - 40mg* per vial - Preservative-Free - 25–1 mL Act-O-Vial® Systems - For ...
-
PRINCIPAL DISPLAY PANEL - 125 mg Vial LabelNDC 0009-0047-25 - 2 mL Act-O-Vial® Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 125 mg* per vial - Preservative-Free - Rx only
-
PRINCIPAL DISPLAY PANEL - 125 mg Vial CartonNDC 0009-0047-22 - Contains 25 of NDC 0009-0047-25 - Single-Dose Vial. Discard unused portion. 25–2 mL Act-O-Vial® Systems - Solu-Medrol® (methylprednisolone sodium - succinate for injection ...
-
PRINCIPAL DISPLAY PANEL - 125 mg Vial Label - Act-O-Vial SystemNDC 0009-0047-27 - Rx ONLY - SOLU-MEDROL® Methylprednisolone Sodium - Succinate for Injection, USP - Single-Dose Vial - For IM or IV Use Only - Discard unused - portion. 125 mg* per ...
-
PRINCIPAL DISPLAY PANEL - 125 mg Vial Carton - Act-O-Vial SystemNDC 0009-0047-26 - Contains 25 of NDC 0009-0047-27 - SOLU-MEDROL® Methylprednisolone Sodium - Succinate for Injection, USP - 125 mg* per vial - Preservative-Free - 25–2 mL ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Vial Label - Act-O-Vial SystemSingle-Dose Vial. Discard unused portion. 4 mL Act-O-Vial® NDC 0009-0003-02 - Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 500 mg* per vial - Preservative-Free - Rx ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Vial Carton - Act-O-Vial SystemNDC 0009-0003-02 - Single-Dose Vial. Discard unused portion. 4 mL Act-O-Vial® Solu-Medrol® (methylprednisolone - sodium succinate - for injection, USP) 500 mg* per vial - For Intramuscular ...
-
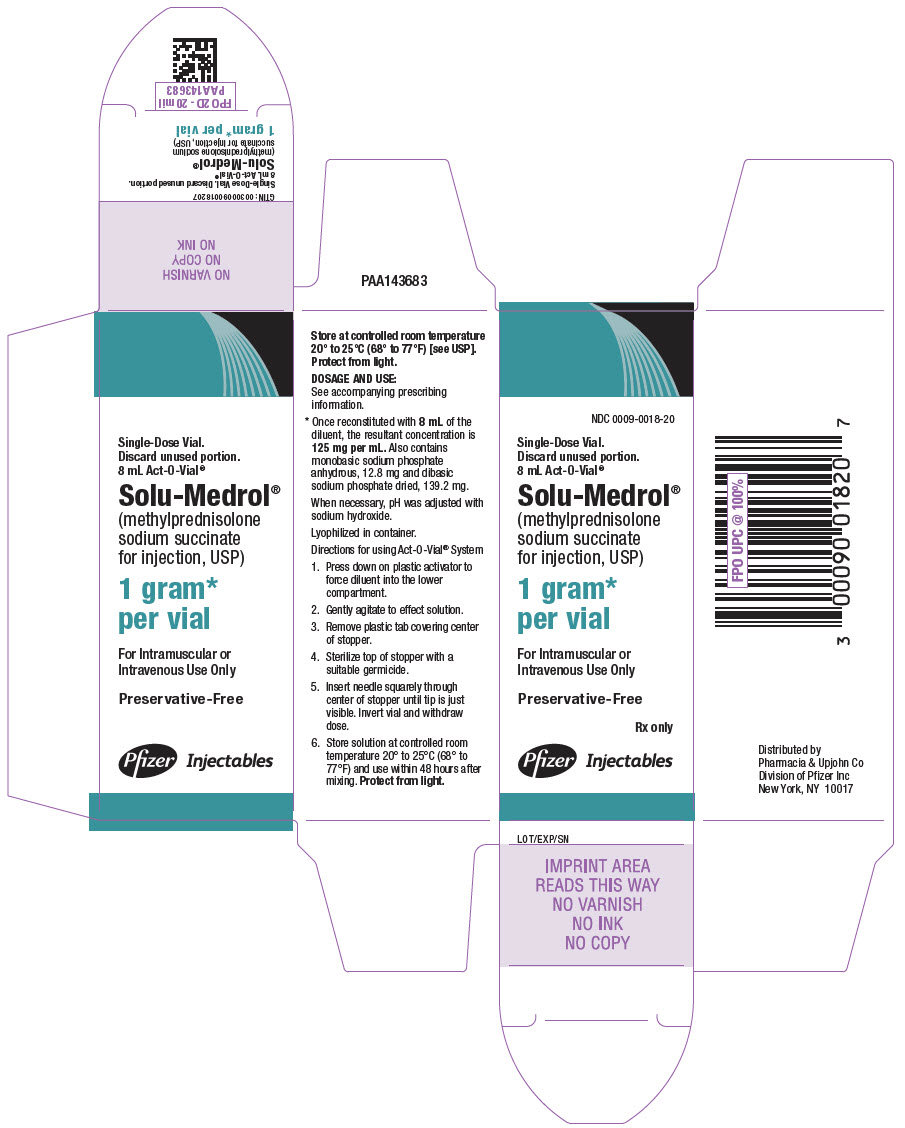
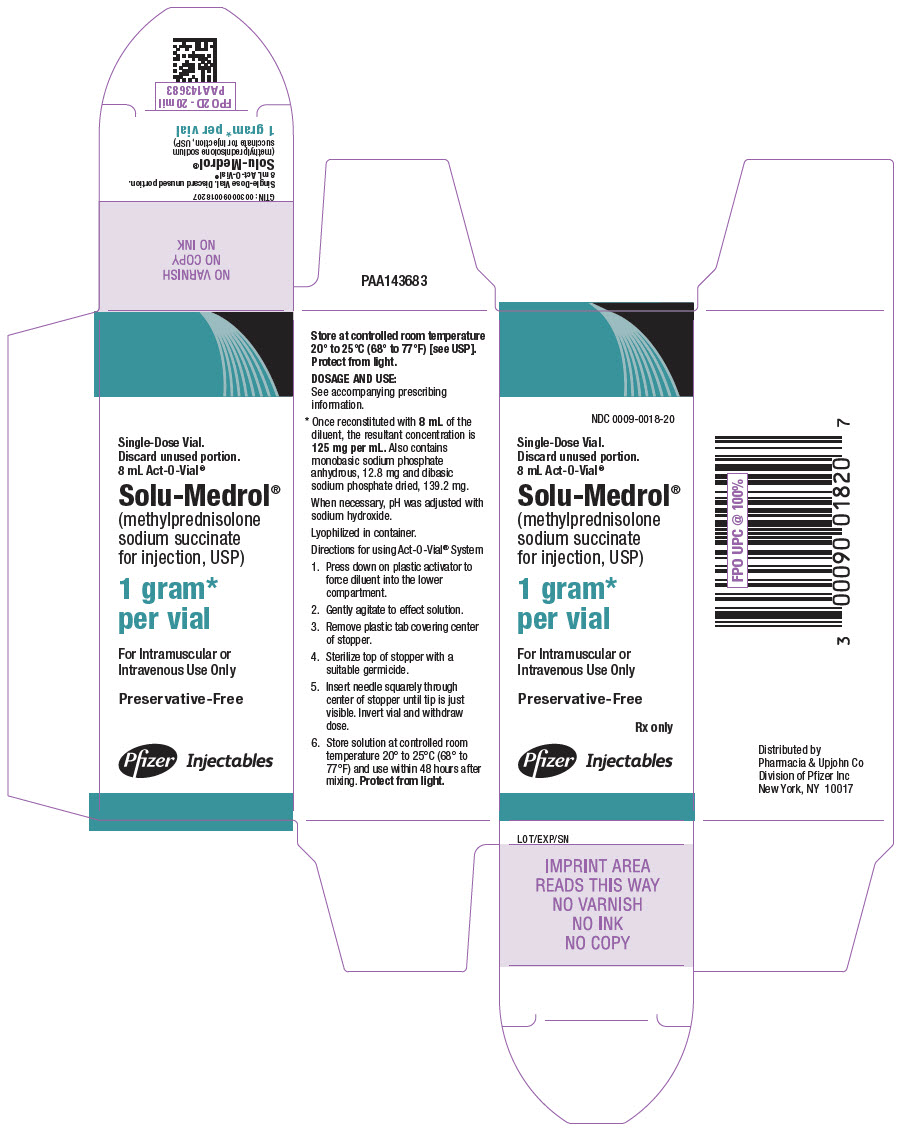
PRINCIPAL DISPLAY PANEL - 1 gram Vial Label - Act-O-Vial SystemSingle-Dose Vial. Discard unused portion. 8 mL Act-O-Vial® NDC 0009-0018-20 - Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 1 gram* per vial - Preservative-Free - Rx ...
-
PRINCIPAL DISPLAY PANEL - 1 gram Vial Carton - Act-O-Vial SystemNDC 0009-0018-20 - Single-Dose Vial. Discard unused portion. 8 mL Act-O-Vial® Solu-Medrol® (methylprednisolone - sodium succinate - for injection, USP) 1 gram* per vial - For Intramuscular ...
-
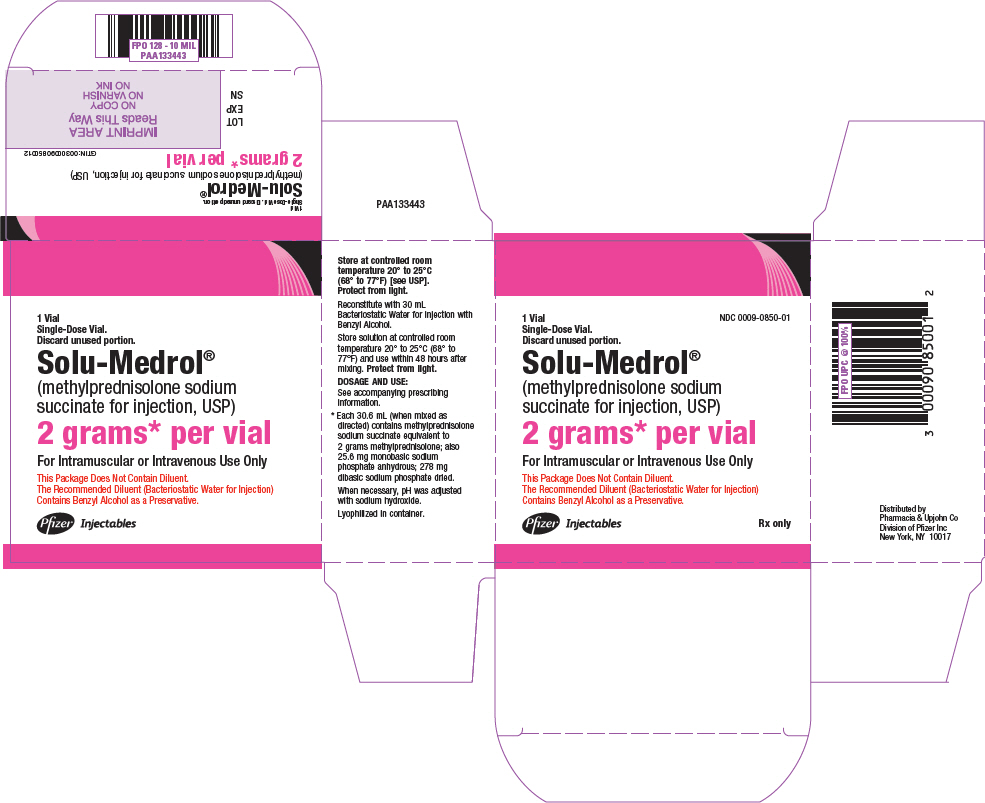
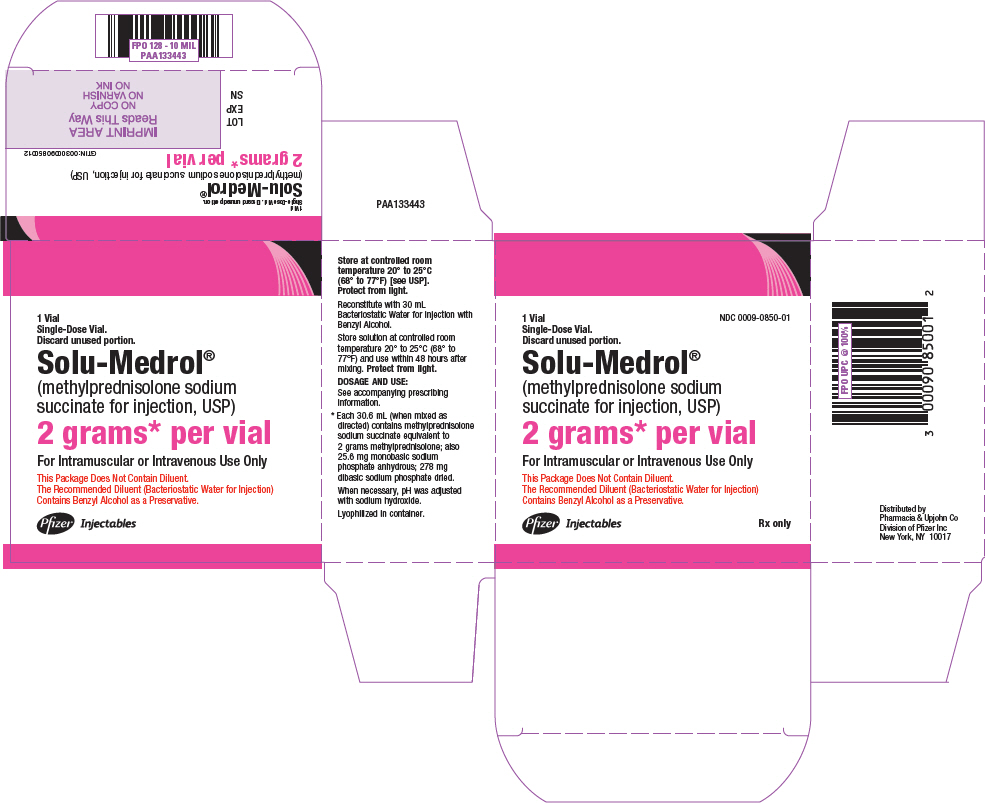
PRINCIPAL DISPLAY PANEL - 2 grams Vial Label - 0850NDC 0009-0850-01 - Single-Dose Vial. Discard unused portion. Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 2 grams* per vial - This Package Does Not Contain - Diluent ...
-
PRINCIPAL DISPLAY PANEL - 2 grams Vial Carton - 0850NDC 0009-0850-01 - 1 Vial - Single-Dose Vial. Discard unused portion. Solu-Medrol® (methylprednisolone sodium - succinate for injection, USP) 2 grams* per vial - For Intramuscular or Intravenous Use ...
-
INGREDIENTS AND APPEARANCEProduct Information