Label: REZLIDHIA- olutasidenib capsule
- NDC Code(s): 71332-005-01
- Packager: Rigel Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REZLIDHIA safely and effectively. See full prescribing information for REZLIDHIA. REZLIDHIA® (olutasidenib) capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME
Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain.
If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGERelapsed or Refractory Acute Myeloid Leukemia - REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients for the treatment of relapsed or refractory AML with REZLIDHIA based on the presence of IDH1 mutations in blood or bone marrow [see Clinical Trials ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 150 mg opaque white capsules imprinted with "OLU 150".
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Differentiation Syndrome - REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Differentiation Syndrome [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on Olutasidenib - Strong or Moderate CYP3A4 Inducers - Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers. Olutasidenib is a CYP3A substrate ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal embryo-fetal toxicity studies, REZLIDHIA may cause fetal harm when administered to a pregnant woman. There are no available data on REZLIDHIA use in ...
-
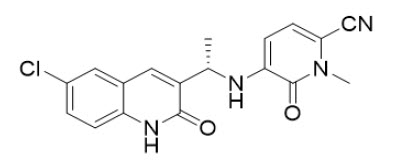
11 DESCRIPTIONOlutasidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor. The chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Olutasidenib is a small-molecule inhibitor of mutated isocitrate dehydrogenase-1 (IDH1). In patients with AML, susceptible IDH1 mutations are defined as those leading ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with olutasidenib. Olutasidenib was not genotoxic in the in vitro bacterial reverse ...
-
14 CLINICAL STUDIES14.1 Acute Myeloid Leukemia - The efficacy of REZLIDHIA was evaluated in an open-label, single-arm, multicenter clinical trial (Study 2102-HEM-101, NCT02719574) in 147 adult patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Capsule StrengthDescriptionPackage ConfigurationNDC Number - 150 mgWhite hard gelatin capsules with black ink print "OLU 150"White high-density polyethylene (HDPE) bottle ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Differentiation Syndrome - Advise patients of the risks of developing differentiation syndrome as early as 1 day ...
-
MEDICATION GUIDEMEDICATION GUIDE - REZLIDHIA® (REZ-LID-EE-AH) (olutasidenib) capsules - What is the most important information I should know about REZLIDHIA? REZLIDHIA may cause serious side effects ...
-
PRINCIPAL DISPLAY PANEL - REZLIDHIA - NDC 71332-005-01 - 150 mg Capsule 30-Count Carton LabelNDC: 71332-005-01 - Rx only - REZLIDHIA® olutasidenib capsules - 150 mg - Dispense the enclosed Medication Guide to each patient. 30 capsules
-
INGREDIENTS AND APPEARANCEProduct Information