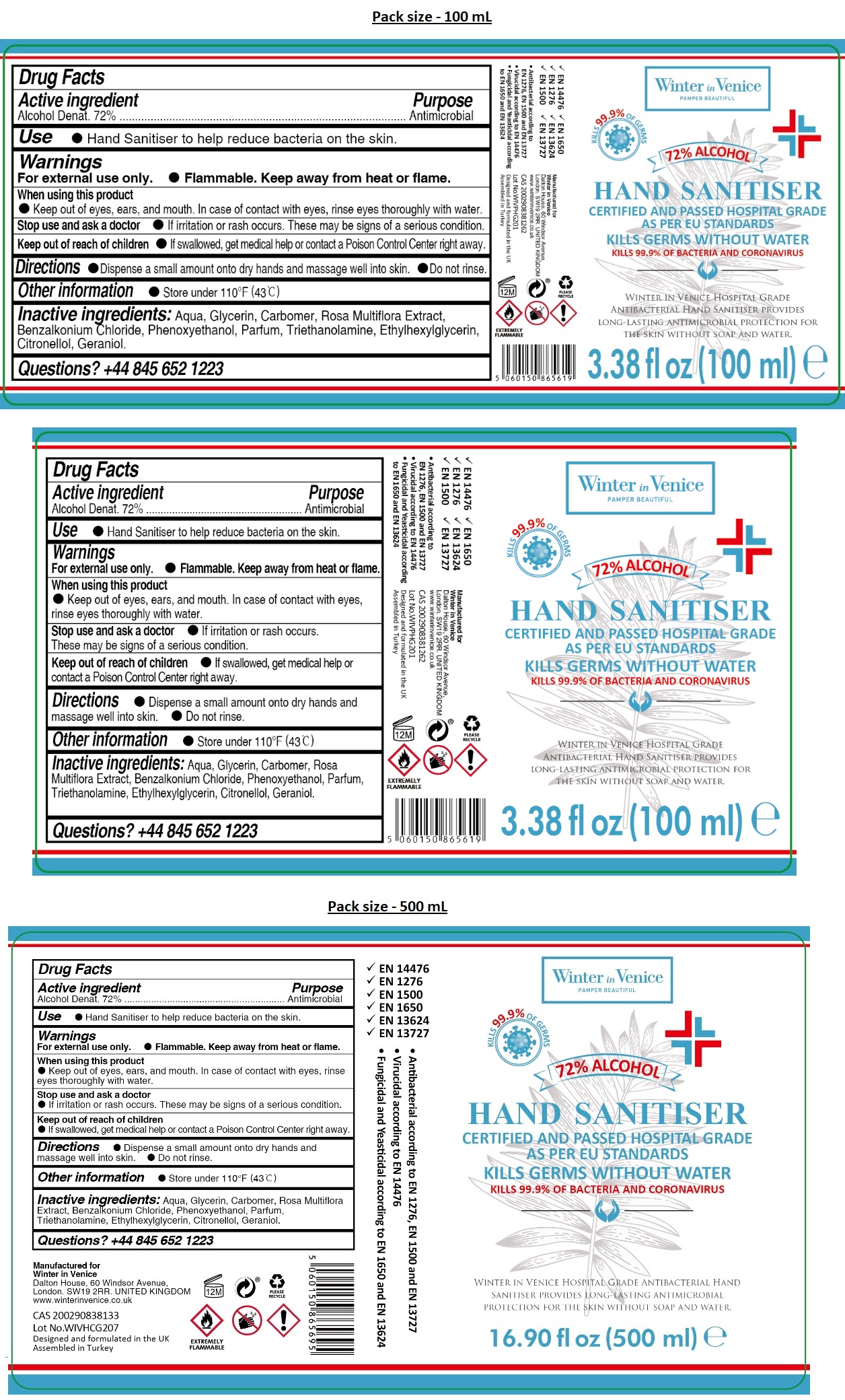
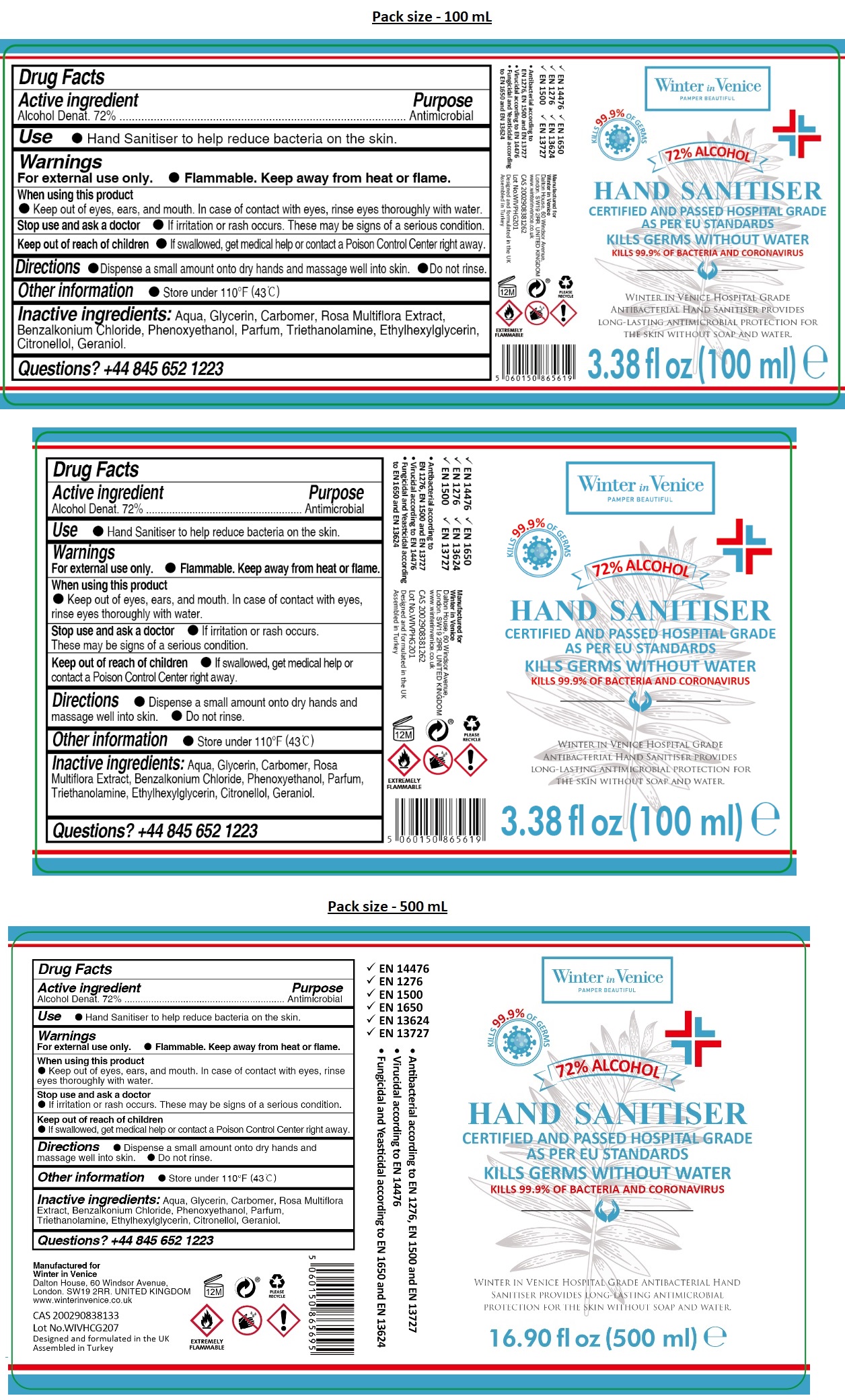
Label: WINTER IN VENICE HOSPITAL GRADE HAND SANITIZER- alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 78609-124-10, 78609-124-50, 78609-124-55 - Packager: WIV Global Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- INDICATIONS & USAGE
- Warnings
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
KILLS 99.9% OF GERMS
CERTIFIED AND PASSED HOSPITAL GRADE AS PER EU STANDARDS
KILLS GERMS WITHOUT WATER
KILLS 99.9% OF BACTERIA AND CORONAVIRUS
WINTER IN VENICE HOSPITAL GRADE ANTIBACTERIAL HAND SANITISER PROVIDES LONG-LASTING ANTIMICROBIAL PROTECTION FOR THE SKIN WITHOUT SOAP AND WATER.
- EN 14476
- EN 1276
- EN 1500
- EN 1650
- EN 13624
- EN 13727
• Antibacterial according to EN 1276, EN 1500 and EN 13727
• Virucidal according to EN 14476
• Fungicidal and Yeasticidal according to EN 1650 and EN 13624
Manufactured for
Winter in Venice
Dalton House, 60 Windsor Avenue,
London. SW19 2RR. UNITED KINGDOM
www.winterinvenice.co.uk
Designed and formulated in the UK
Assembled in Turkey
- Packaging
-
INGREDIENTS AND APPEARANCE
WINTER IN VENICE HOSPITAL GRADE HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78609-124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ROSA MULTIFLORA WHOLE (UNII: XZ57EQ287T) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) PHENOXYETHANOL (UNII: HIE492ZZ3T) TROLAMINE (UNII: 9O3K93S3TK) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78609-124-10 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/02/2020 2 NDC:78609-124-50 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/02/2020 3 NDC:78609-124-55 5000 mL in 1 CAN; Type 0: Not a Combination Product 05/26/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/26/2020 Labeler - WIV Global Limited (211563238)